Patogenní bakterie, jako jsou Haemophilus influenzae typu B, meningokoky, pneumokoky a tyfoidní salmonely, mají kapslovou strukturu, která může způsobit invazivní infekce u dětí. Kapslové polysacharidy jsou důležitými faktory způsobujícími tyto bakteriální infekce a jsou cílovými antigény pro vývoj vakcín. Vakcíny založené na bakteriálních polysacharidech zahrnují polysacharidové vakcíny a konjugované vakcíny. Polysacharidové vakcíny používají polysacharidové antigény jako aktivní složky.
Konjugované vakcíny vznikají spojením polysacharidů s nosiči, jako jsou toxoidy, které mohou zvýšit ochranný účinek vakcíny.
Yaohai Bio-Pharma má více než deset let zkušeností s mikrobiálním CDMO. Na základě GMP laboratoře s biologickou bezpečností úrovně 1 (BSL-1) a biologickou bezpečností úrovně 2 (BSL-2) nabízíme kompletní řešení pro vývoj mikrobiálních šťáv, fermentaci, extrakci a čištění polysacharidů a nosných bílkovin, konjugaci a sterilní vyplňování.
Podle individuálních požadavků zákazníků poskytujeme zákazníkům meziprodukty, látku léčivu (DS, API) nebo přípravek (DP), které splňují kvalitní normy, stejně jako záznamy o produkci podle GMP a testovací hlášení.
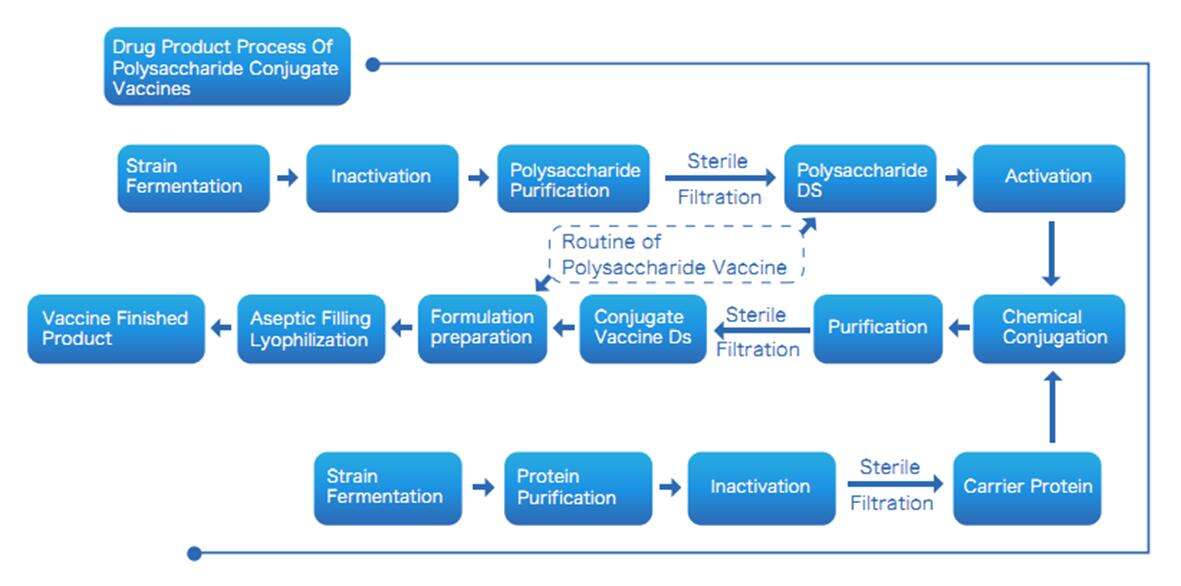
Výrobní proces vakcín na bázi konjugovaných polysacharidů
Dodané výsledky
|
Třída
|
Dodávané výsledky
|
Specifikace
|
Aplikace
|
|
GMP, BSL-1/BSL-2
|
Přechodná látka
|
Polysacharidový antigen
|
Investigační nový lék (IND),
Schválení klinického výzkumu (CTA),
Zásobování klinickými studiemi,
Žádost o licenci biologického přípravku (BLA),
Komercní zásobování
|
|
Nosný protein
|
|
Látka pro léčivo
|
Konjugovaná vakcína
|
|
Lék
|
Fialy (kapalné)
|
|
Fialy (lyofilizované)
|
|
Jiné dávkové formy
|

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN


