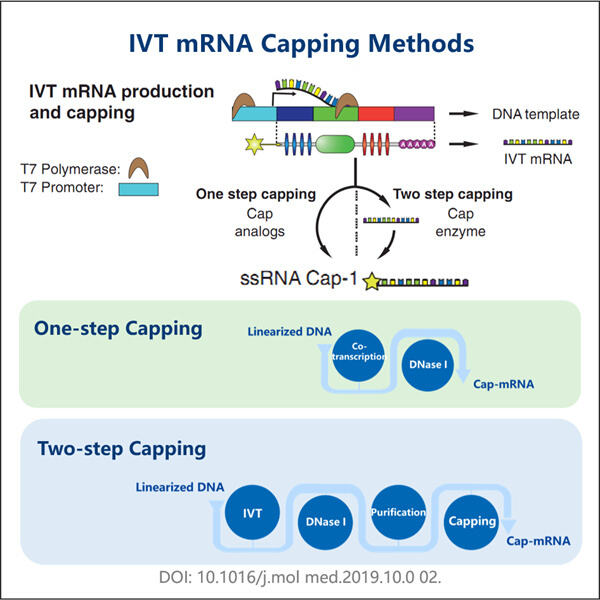
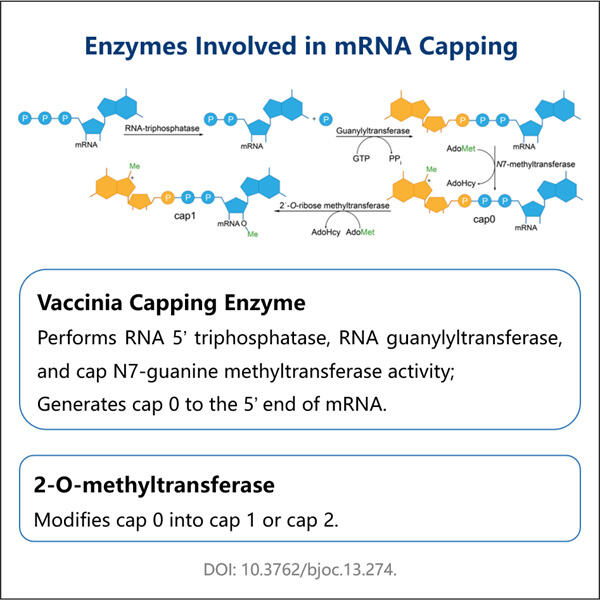
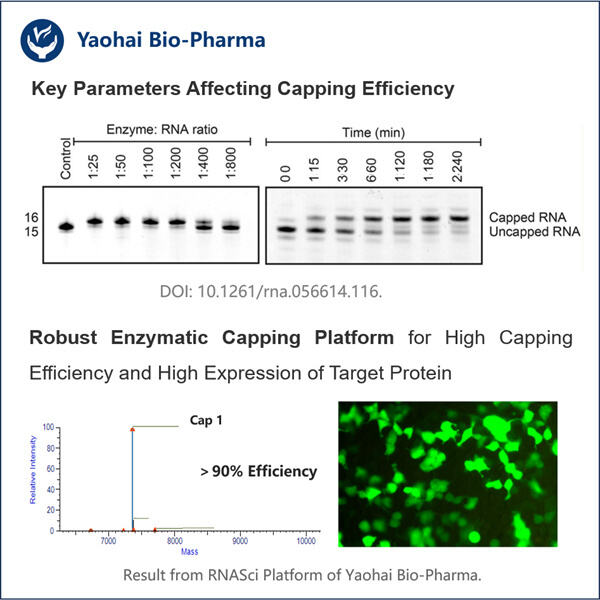
Haben Sie schon einmal von mRNA gehört? Sie steht für Messenger RNA. mRNA ist ein wichtiger Bestandteil unserer Zellen und hilft bei der Bildung von Proteinen. Proteine sind wie kleine Maschinen, die verschiedene Arbeiten in unseren Körpern ausführen. Der Grund, warum Wissenschaftler sich für mRNA interessieren, ist, dass sie so etwas wie eine übersichtliche Kurzinformation sein kann, die uns sagt, was eine Zelle tut und wie das Organ gesund bleibt. Es ist jedoch schwierig, mRNA allein zu studieren, da sie sehr empfindlich ist und leicht abbauen kann. Dies ist ein deutliches Zeichen dafür, dass mRNA unbrauchbar für Forschungen werden kann und praktisch nicht weiter analysiert werden kann, wenn sie nicht sorgfältig behandelt wird. Hier kommt unser neuer Ansatz ins Spiel. Bislang wurde mRNA gehasst und nach der Verwendung vollständig abgebaut, aufgrund ihrer instabilen Natur. Deshalb haben wir einen anderen Weg entwickelt, um die mRNA für Wissenschaftler auf der ganzen Welt zu schützen. Es wird als enzymatisches Capping-Protokoll für mRNA bekannt und zeigt einen neuen Weg, wie Forscher ihre Forschungen durchführen.
Das auch, mögen Sie fragen, was ist das enzymatische Kapierprotokoll für mRNA? Dies ist eine einzigartige Methode, um ein mRNA-Molekül zu kapieren, ähnlich wie beim Produkt von Yaohai CDMO für mikrobielle Fermentation . Diese Kappe ermöglicht es der mRNA, stabil zu bleiben und nicht so schnell zu degenerieren. Bevor es diesen neuen Ansatz gab, mussten Wissenschaftler komplexe Chemie verwenden, um das Ende der mRNA zu markieren. Die alte Methode war langsam und es konnte schwierig sein, sie richtig auszuführen. Manchmal konnten die Wissenschaftler es einfach nicht richtig machen. Nun haben wir versucht, diesen Prozess mit unserem neuen Protokoll zu vereinfachen.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN