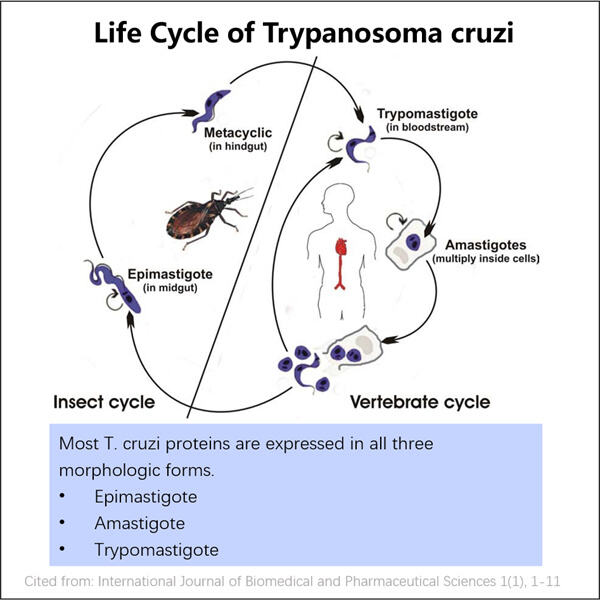
-
รูปแบบ
- โปรตีน
- นิวคลีอิกแอซิด
- ชีวภัณฑ์สัตว์
-
วัคซีน
- วัคซีนดีเอ็นเอ
- วัคซีน mRNA
- วัคซีนย่อยที่สังเคราะห์ขึ้นใหม่
- วัคซีนบำบัดที่ใช้โปรตีน
- วัคซีนเวกเตอร์ไวรัส
- โปรตีนพาหะ รีคอมไบแนนต์
- วัคซีนเชื้อเป็นอ่อนแรง (แบคทีเรีย)
- วัคซีนเวกเตอร์แบคทีเรียแบบมีชีวิต
- วัคซีนเชื้อตาย (แบคทีเรีย)
- วัคซีนโพลีแซคคาไรด์หรือวัคซีนคอนจูเกต
- วัคซีนโทไซด์
- โปรตีนพาหะ แยกได้
- สารเสริมฤทธิ์วัคซีน
- ยาความงาม
- สารตรวจวินิจฉัย
- วัตถุดิบและสารละลาย
- ผู้ให้บริการ CDMO ด้านจุลชีพ
- การเติมและเสร็จสิ้น
- วัคซีน
- IVT RNA
- บัยโอสังเคราะห์ APIs
- ข่าวสาร
- ดาวน์โหลด
- เกี่ยวกับ
- ติดต่อ

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN