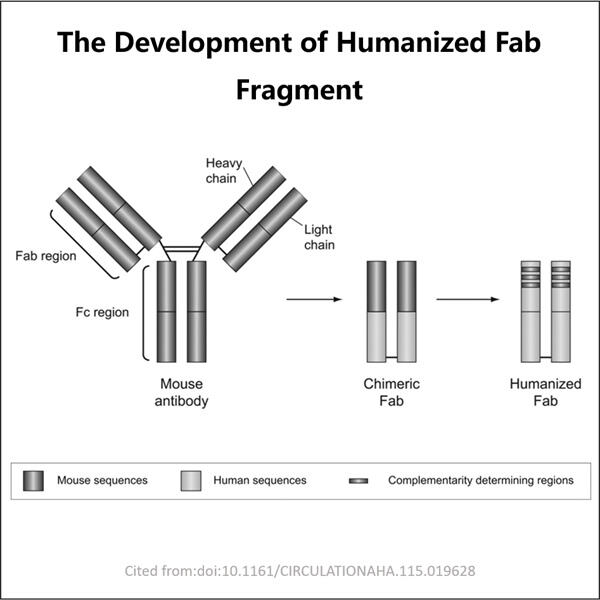
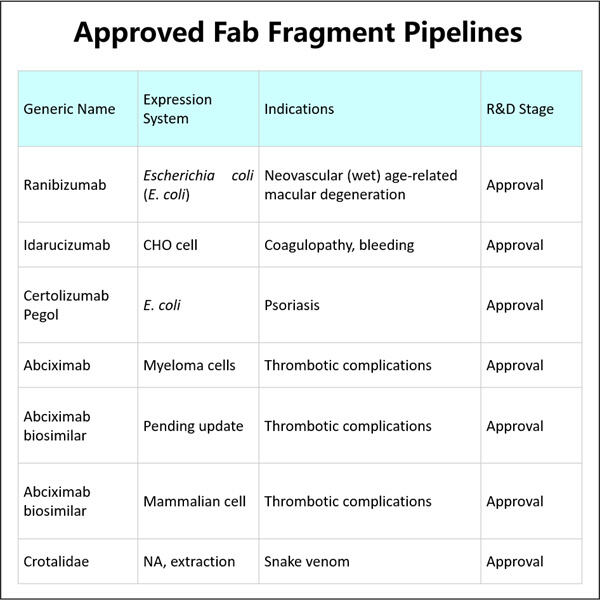
Wir legen großen Wert darauf, sichere und wirksame Medikamente bei Yaohai herzustellen. Deshalb nutzen wir oft das, was als GMP (Good Manufacturing Practices) bekannt ist. Dies sind eine Reihe von Leitlinien, die uns dabei helfen, jeden konstruktiven Schritt bei der Herstellung unserer Produkte auf die bestmögliche Weise auszuführen. Demnach bedeuten diese Leitlinien, dass wir ein Auge für die Details haben, was dazu beiträgt, unsere Prozesse ordentlich zu halten und unsere Produkte sicher für Patienten zu machen.
Da neue Krankheiten auftreten und Menschen früher Behandlung benötigen, haben wir effektivere Methoden zur Herstellung von Fab-Fragmenten entwickelt, um ihren Anforderungen gerecht zu werden. Dadurch können wir hohe Qualität produzieren, GMP GLP-1-Herstellung schnell und zuverlässig, was unsere Arbeit verbessert. Auf diese Weise erreichen wir Ärzte und Patienten schneller – super wichtig in Anbetracht des medizinischen Kontextes.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN