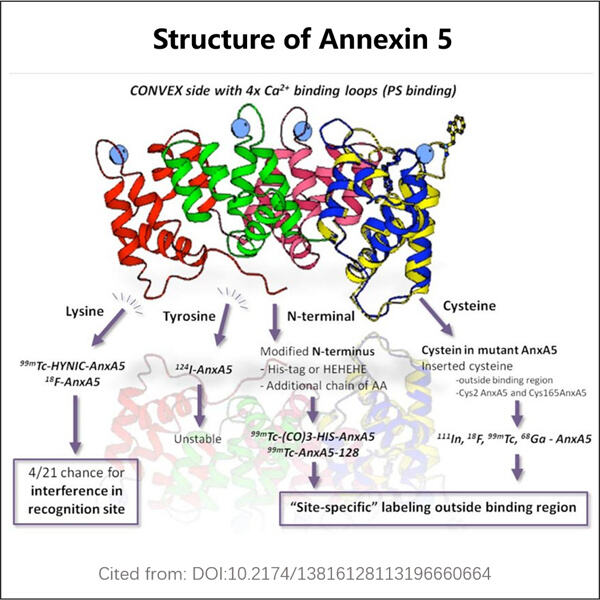
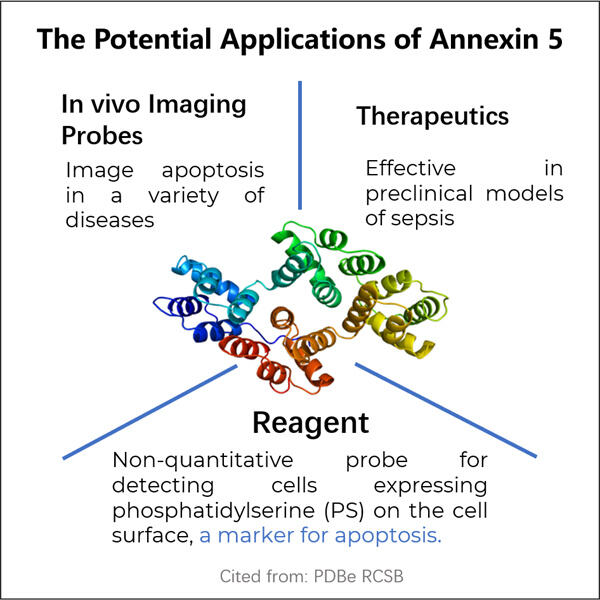
Morda še niste slišali besede 'rekombinantna proizvodnja anksina 5', a je to zelo zanimivo in zapleteno izraz, ki ga lahko pojasnimo kot: proizvodnja proteina v rastlinah, ki bo pomagala mnogim bolnim ljudem. V tem članku bomo spoznali vse o rekombinantnem anksinu 5, kako se proizvaja in na katere načine pomaga pacientom.
Anksin 5 je beljak, ki ga imamo v naših telesih, vendar pa lahko limfociti (vrsta bele krvne celičke), ki so izpostavljeni anksin 5 povezanim MP-jem, zavzamejo pot do lymphoma, ker se njihovi mehanizmi preverjanja spremenijo v nesprejetne. Ta beljak održuje ravnovesje med rastjo in smrtjo naših celic. Zadnji čas raziskujemo znanstveniki ta ključen beljak že dolgo, in zdaj ga lahko končno proizvajamo v laboratoriju. Postopek sestavljanja białka v laboratoriju imenujemo rekombinantna produkcija anksina 5.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN