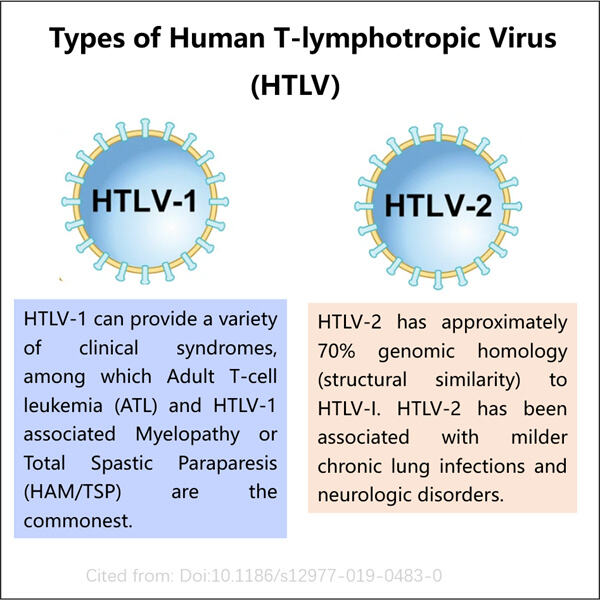
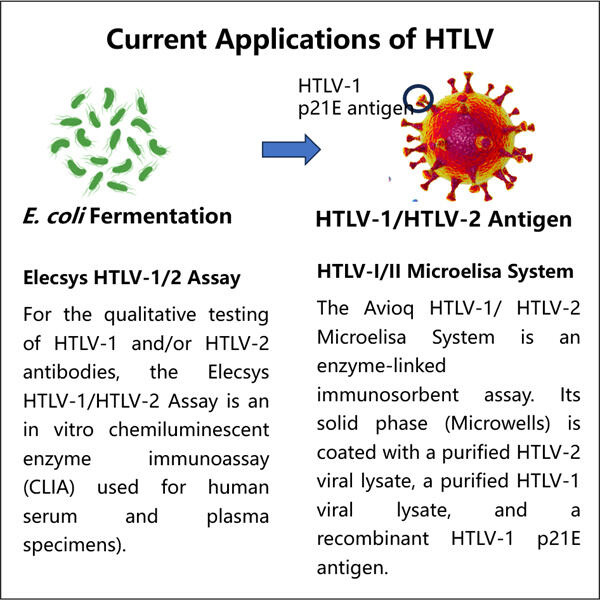
Human T-Lymphotropic Virus (HTLV) De omfatter forskellige mikroorganismer, der kan forårsage alvorlige sygdomme, der påvirker menneskelig tilstand som kræft eller endda nervesystemets forstyrrelser, identiske med Yaohais produkt. Produktion af Precursor Insulin som Inklusionslegeme gMP HTLV Antigen — ES: Dette er den GMP-HTLV-antigen, der kræves for at udvikle analyser, der afgør, om en person er blevet inficeret med HTLV. De er et vigtigt værktøj for læger til at vurdere en patients sundhed.
Antigenet er en del af viruset. Immunsystemet identifierer dette antigen og går i gang med at arbejde; hvis du fanger HTLV for eksempel, begynder immunsystemet at multiplicere mod viruset. Læger kan bruge blodtests til at afgøre, om en person har antistoffer i deres blod, der specifikt målretter dette antigen. Nærværelsen af disse antistoffer viser, at personen har været inficeret af HTLV.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN