Fortschritte in der Biopharmazeutischen Forschung
Biologika sind Schlüsseltreiber zielgerichteter Forschung zur Entwicklung neuer Medikamente, die menschliches Leid lindern. Biopharmazeutika sind Medikamente, die mit lebenden Zellen anstelle von Chemikalien hergestellt werden. Biopharmazeutika oder Biologika sind eine Art biologischer Produkte, die Impfstoffe einschließen, die helfen, Krankheiten vorzubeugen, und Behandlungen, die helfen, Erkrankungen zu bekämpfen. Um innovative und verbesserte Medikamente zu entwickeln, ist es wichtig, die Mechanismen dieser Biologika zu verstehen.
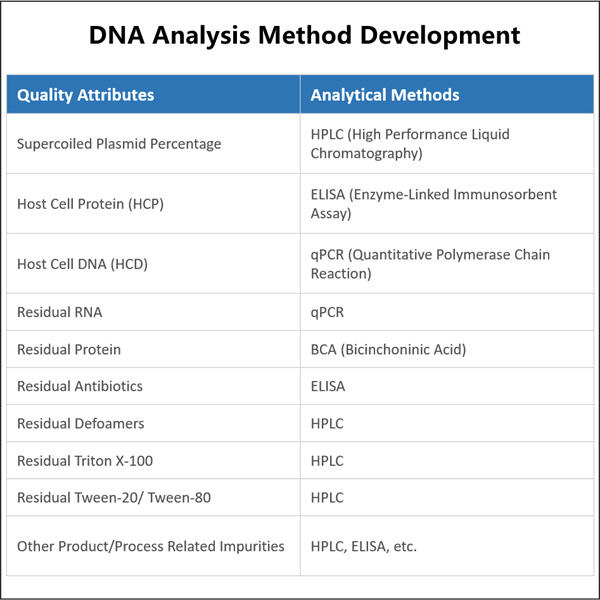
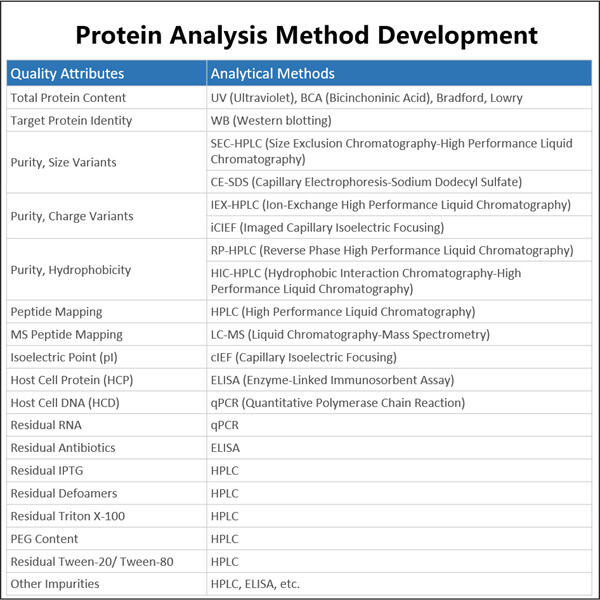
Wir sagen gerne, dass bei Yaohai unsere Gehirnlichter 24/7 brennen, um darüber nachzudenken, wie wir bessere Biopharmazeutika herstellen können. Dies beinhaltet die Untersuchung lebender Zellen, die die Grundlage der Biologika bilden. Da verschiedene Zellen stattdessen unterschiedliche Arten von Biologika produzieren, Analytische Methoden für Plasmid-DNA das Verständnis der Art von Zellen, die wir in diesen Transplantaten haben, wird uns helfen zu erfahren, wie effizient dieses Medikament sich verhält. Diese Informationen unterstützen uns dabei, wirksamere Medikamente mit weniger Nebenwirkungen zu entwickeln.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN