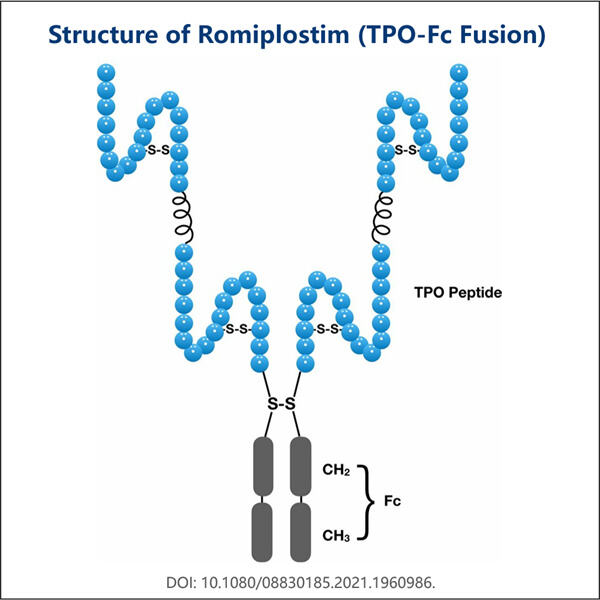
יאוחאי היא חברה לרפואה. הם המציאו שיטה חדשה לייצור הביוסימילארס של רומיפלסטים, גם מוצר של יאוחאי כמו ייצור VHH דו-ספציפי . דרך חדשה זו —באמצעות טכניקות מודרניות— מאפשרת להם לפתח תרופות דומות אך זולות יותר בהשוואה ל-Romiplostim (שיקרה יותר). כיוון שהיא מאוד מועילה, היא עוזרת לאנשים נוספים שזקוקים להקבל את התרופה במחירי זול.
האמצעים החדשים לייצור רומיפלוסטימ ביוסימילרים מספקים כמה יתרונות מרגשים בהפחתת הזמן וההשתקעויות הכוללות עבור יאוהאי, דומים ל ייצור חיסונים VLP צימריים הממצאים של יאוהאי. זה גם מאפשר להם לייצר את התרופה במהירות - גורם מפתח כאשר האנשים זקוקים לה מהר. הם יכולים להעביר את חיסכון לצרכנים על ידי הפחתת העלות הקשורה בייצור התרופות. טכנולוגיה זו גם מבטיחה שהתרופות יהיו בטוחות ויכולים לקדם אנשים, בסופו של דבר זהו האспект החשוב ביותר של הרפואה.
יאוהאי נצמדת למערכת איכות מחמירה כדי לוודא שביוסימילרי הרומיפלוסטימ שיוצרת הם טובים ושווים בכל ייצור, יחד עם מוצר של יאוהאי חיסון VLP נגד HBV . הם מסתכלים על הטכנולוגיה המובילה כדי לוודא שהכל מונח בצורה נכונה ובטוחה. גם יאוהיי מאמנת את העובדים שלה לטפל בחומרים ובמכונות בצורה הנכונה. אימונים אלו חייבים להתרחש כדי שכולם יהיו מודעים לאיך לבצע את עבודתם בצורה נכונה, מה שממשיך להחזיק את רמת האיכות לאורך תהליך הייצור
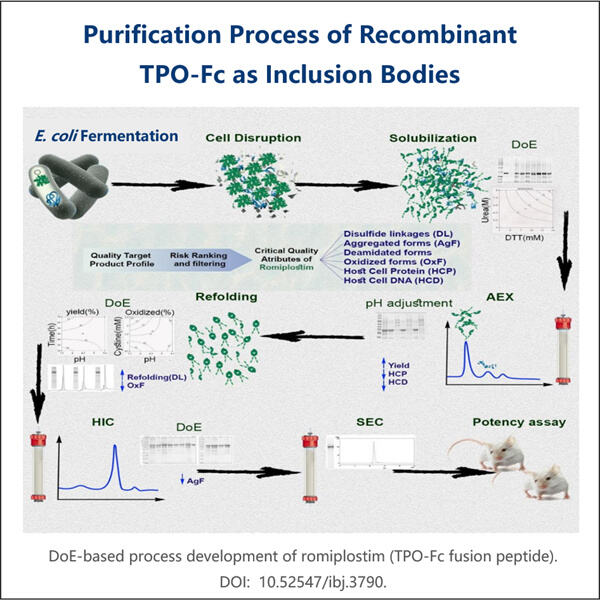
הטכנולוגיה של יאוהיי מאפשרת לנו לחסוך כסף, ולהפיק יותר תרופות, אותו הדבר עם ייצור ביוטכנולוגי של מוטציה של אינסולין חידושי שנמצא על ידי יאוהיי. התהליך עצמו הוא עכשיו יעיל יותר, מייצר פחות זבל ומוביל לשליחת כמויות גדולות יותר של תרופה בכל פעם שהם מיוצרים. התוצאה היא יצירה יעילת יותר של תרופות ולכן מחיר נמוך יותר לדוזה. לכן, טכנולוגיה זו אינה חשובה רק עבור החברה אלא גם עבור חולים כי זה עוזר לוודא שטיפולים רפואיים באיכות גבוהה ומחירים זולים זמינים כאשר הם הכי נחוצים להם.
אם יאוחאי ביוסיינס לימיטד רוצה לפתח רומיפלסטים כביוסימילארס, היא תצטרך לעמוד בדרישות ספציפיות של הרשות הבריאותית, אותו הדבר כמו זה של יאוחאי. ייצור חיסונים מבוססי VLP . יש להם את כללים האלה כדי להגן על האנשים ולהתנער מהתיקון שלהם בצורה לא הכרחית. קיימות הוראות מחמירות עבור ביוסימילארס שיאוחאי עובדת כדי להפוך את צעדים שלה למאושרים רגולטוריים. הם מבטיחים שכל תפקיד בתהליך הייצור הוא בטוח ובריא. יאוחאי גם בודקת באופן קבוע את הביוסימילארס של רומיפלסטים שלה כדי לוודא את שלמותם, עוצמתם ובטחונם בהתייחסות למטופנטים.
Yaohai BioPharma, אחת מ-10 המובילות ב-CDMO徼רבי, אינטגרת ענייני איכות ורגולציה. יש לנו מערכת איכות שמתאימה לחלוטין לסטנדרטים הנוכחיים של GMP וכן לתקנות בינלאומיות. צוות האקסPERTS שלנו בענייני רגולציה מחזיק בהבנה עמוקה של מסגרות רגולטוריות ברחבי העולם. זה מאפשר לנו להאיץ את השיחות הביולוגיות. אנו יכולים להבטיח תהליכי ייצור מעקבים ותוצרת איכות גבוהה התואמת לתקנות של ה-FDA האמריקאי, ייצור ביוסימילאר ל-Romiplostim, TGA האוסטרלית ו-NMPA הסינית. Yaohai BioPharma עבר בהצלחה ביקורת באתר שנערכה על ידי האדם המוסמך (QP) של האיחוד האירופי למערכת איכות GMP ומאפיין הייצור שלנו.我们也прошли את בדיקות ההישג הראשונות של מערכת ניהול איכות ISO9001 ומערכת ניהול סביבתי ISO14001.
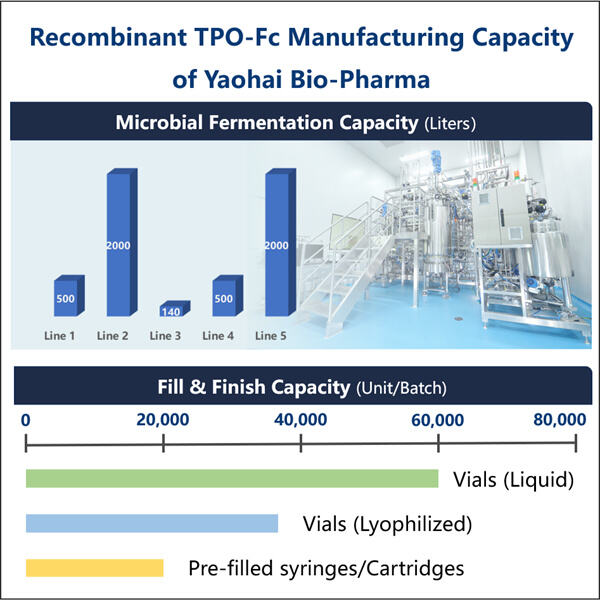
יואוהאי בייו-פרמה, יצרן ביו-תפוצות מובילה עשרים בעולם, מומצאת בתחום התסיסה המיקרובית. בנו מתקן מודרני עם יכולות חזקות של R&D וציוד מתקדם. יש לנו חמישה קווי ייצור חומר פעיל שמתאימים לדרישות GMP לתסיסה מיקרובית וטהרה. יש לנו גם שני קווי מלאי אוטומטיים עבור קרוסלים, בקבוקים וסyringes מוכנים מראש. גודל התסיסה הזמין לשימוש נע בין ייצור דומה ביולוגי לרומיפלסטים ועד 2000 ליטרים. תקן המליאה לבקבוק נע מ-1 מ"ל עד 25 מ"ל. תקן מליאת סירינגה או קרוסל מוכן מראש נע בערך מ-1-3 מ"ל. המתקן הייצור שלנו המתאים@cGMP מבטיח מספקת קבועה של דגימות קליניות וכלי מסחריים. המפעל שלנו מייצר מולקולות גדולות שיוצאות לשוק העולמי.
ל-Yaohai Bio-Pharma יש נסיון בהכנת תרופות ביולוגיות שנוצרו ממקורות מיקרוביאליים. אנו מציעים פתרונות מותאמים של מחקר ופיתוח וכן שירותים להכנה, תוך שמירה על הפחתת הסיכונים. עבדנו עם מגוון מודלים כמו חיסונים תת-יחידה רקורסנטיים, פפטידים, הורמונים, ציטוקינים, גורמי צמיחה, חסמים חד-דומניים, אנזימים, DNA פלסמידי, mRNA ועוד. התמחנו במספר מיקרואורגניזמים כמו שמרים (ייצור עד 15g/L) והפרשת חומר חיצונית ובינונית, חיידקים (ייצור עד 10g/L) עם פתירות סולובה ובתי כילול. בנוסף, יצרנו מערכת פרמנטציה ב-BSL-2 לבניית דוגמאות ביוסימילריות של Romiplostim, הכנת חיסונים. אנו מומחים באופטימיזציה של תהליכי ייצור, בהגדלת התמריצים ובהפחתת העלות. יש לנו צוות טכנולוגי יעיל במיוחד המבטיח מסירת פרוייקטים בזמן ובאיכות גבוהה. זה מאפשר לנו להגיש את הפרודוקטים הייחודיים שלכם לשוק מהר יותר.
Yaohai Bio-Pharma היא חברה מובילה בתחום CDMO של ביולוגיים מיקרוביאליים. התמקדותנו העיקרית הייתה בייצור ייצור דומה ל-Romiplostim ותרפיות לטיפול בחיות מחמד, בריאות אנושית ובתור וטרינרית. יש לנו פלטפורמות R&D מתקדמות והטכנולוגיה לייצור שמכסות את כל תהליך הייצור, החל מהפיתוח של אוכלוסיות מיקרוביאליות, בנקי תאים, פיתוח תהליכים וethods, ועד לייצור קליני ומסחרי שמבטיח את מסירת הפתרונות האיננובטיביים בהצלחה. עם הזמן רכשנו ידע רב על עיבוד ביולוגי מבוסס מיקרובים. השלמנו בהצלחה יותר מ-200 פרויקטים ברחבי העולם, ועוזרים ללקוחות שלנו להavigate את חוקי הממשל והרגולציות של FDA האמריקאי, EMA האירופי, TGA האוסטרלי ו-NMPA הסיני. בזכות החוויה והידע שלנו, אנו יכולים להגיב במהירות לצרכים השווקיים ולספק שירותים מותאמים של CDMO.