نوترکیب به معنای ترکیب اجزای مختلف است. PTH- Horm1 پاراتیروئید: هورمونی که وظیفه دارد در بدن ما برای تنظیم سطح کلسیم کار کند. در مرحله بعد، بنابراین همه ما می دانیم که کلمه "تولید" چیست، ساختن چیزی از ماشین آلات و فناوری. با ترکیب همه این کلمات، Yaohai را به ما می دهد تولید زیستی انسولین نوترکیب خوک و می توان آن را ساختن هورمون پاراتیروئید با استفاده از ماشین ها با اتصال هوشمندانه آن قطعات تعریف کرد.
PTH یک نوترکیب جدید است که توسط دانشمندان در طول سال ها ساخته شده است. این توسط موجودات کوچکی به نام باکتری استفاده می شود و از پروتئین خاصی برای تولید هورمون پاراتیروئید استفاده می شود. یکی از این رهبران بازار Yaohai است. آنها یک فرآیند بدیع و بدیع را برای PTH نوترکیب توسعه دادند.
برای اینکه باکتریها پخته شوند، ابتدا مقداری DNA (کتاب آشپزی جادویی که به حشرات کوچک میگوید چه موقع و کجا روشن شوند) را جمع میکنند، سپس این DNA را به باکتری تبدیل کردند. سپس این باکتری ها را در آزمایشگاه پرورش می دهند. یائوهای تولید پپتید نوترکیب GMP هورمون PTH را آزاد می کند، که باعث می شود آنها مانند ما تولید مثل کنند و PTH بیشتری تولید کنند. بنابراین کیمیاگری روشی موثر برای دریافت مقدار زیادی از این هورمون است.
علاوه بر این، این منبع علاوه بر این، به تولید تکنیکهای مقرونبهصرفهتر با کیفیت اقلام PTH برای مصرفکنندگان کمک میکند. PTH از طریق روشهای معمولی تولید میشود که گران هستند زیرا بر اساس PTH حیوانات و انسان هستند. این می تواند یک رویکرد وقت گیر و منابع فشرده باشد، به خصوص زمانی که مجبور هستید مقادیر زیادی از هورمون را سنتز کنید.
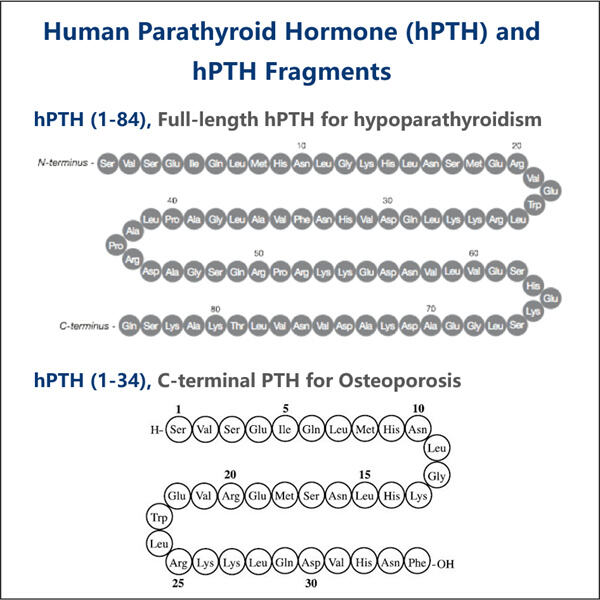
در مقابل، ساخت PTH نوترکیب دشوار و گران است، با این تفاوت که هورمون مذکور را از طریق استفاده از باکتری می سازد. فرآیند جدید تولید PTH نوترکیب فرآیندی سریعتر و سادهتر است. این به نوبه خود به پزشکان مربوطه اجازه میدهد تا درمان PTH خود را با قیمتی کاهشیافته ارائه دهند، زیرا هزینههای مربوط به رسیدگی و تهیه/اداره آن را متحمل نمیشوند. این بدان معناست که افراد بیشتری می توانند به دنبال دریافت کمکی باشند که حق دارند و نگران هزینه های بسیار پرهزینه نباشند.
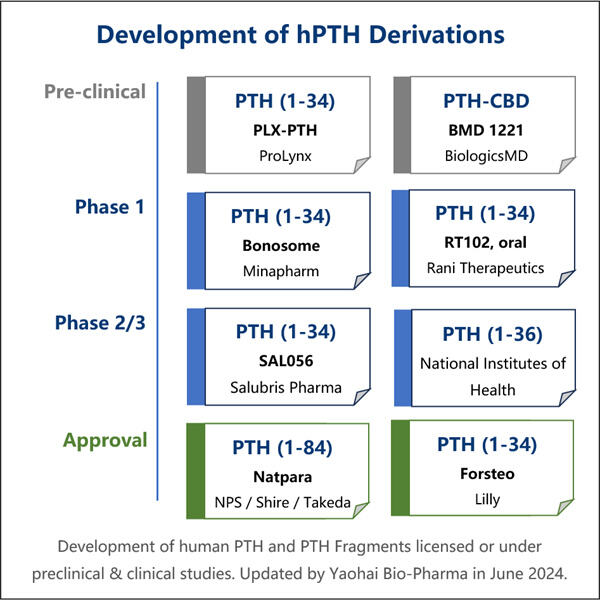
این را می توان یک پیشرفت در زمینه پزشکی نامید و در آنجا ما تولید PTH نوترکیب را داریم. این روش راه بسیار سادهتر و ارزانتری برای ساخت هورمونهای PTH ضروری برای درمانها فراهم میکند. اکنون، بیماران میتوانند درمانهای خود را بدون نگرانی در مورد گرانی آن انجام دهند. همانطور که اخبار می گویند، این موضوع باعث آسودگی خاطر بسیاری از افرادی شد که به چنین مراقبتی نیاز دارند.

PTH نوترکیب نیز قابل پیش بینی است که من را به مزیت اصلی بعدی می رساند. بر خلاف PTH معمولی (برای مثال استخراج شده از منابع متعدد و در نتیجه اغلب متفاوت است)، بنابراین نتایج ممکن است با PTH سنتی متفاوت باشد. به یاد داشته باشید که Yaohai تولید پروتئین پارکین نوترکیب در محیط کنترل شده تولید می شود و از این رو نتایج با کیفیت هر بار تضمین می شود. بیماری که نیاز به درمان منظم دارد می تواند ارزش واقعی را در این ثبات ببیند.
Yaohai Bio-Pharma پیشرو در CDMO تولید PTH نوترکیب است. تمرکز اصلی ما تولید واکسن های میکروبی و درمانی برای درمان حیوانات خانگی، سلامت انسان و دامپزشکی بوده است. ما دارای پلتفرمهای پیشرفته RD و فناوری تولید هستیم که کل فرآیند تولید از مهندسی سویههای میکروبی گرفته تا پردازش بانک سلولی و طراحی روش تا تولید بالینی و تجاری را پوشش میدهد و اطمینان حاصل میکند که میتوانیم از ارائه موفقیتآمیز پیشرفتهترین راهحلها اطمینان حاصل کنیم. ما مقدار زیادی دانش در زمینه میکروبی پردازش زیستی جمع آوری کرده ایم. بیش از 200 پروژه با موفقیت تکمیل شده است و ما به مشتریان خود کمک می کنیم تا از مقرراتی مانند FDA ایالات متحده و همچنین اتحادیه اروپا EMA پیروی کنند. ما همچنین به آنها کمک می کنیم تا TGA استرالیا و NMPA چین را هدایت کنند. ما به دلیل تجربه و تخصص خود قادر به پاسخگویی سریع به نیازهای بازار و ارائه خدمات CDMO سفارشی هستیم.
شرکت نوترکیب PTH در تولید مواد بیولوژیکی که از میکروارگانیسمها مشتق شدهاند، تجربه دارد. ما راهحلهای RD و همچنین تولیدی را ارائه میکنیم، در حالی که خطر را به حداقل میرسانیم. ما با تکنیکهای مختلفی مانند زیرواحدهای سلولی نوترکیب واکسنها (از جمله پپتیدها)، فاکتورهای رشد، هورمونها و سیتوکینها آزمایش کردهایم. ما در میکروارگانیسم های متعددی مانند ترشح خارج سلولی و درون سلولی مخمر (بازدهی تا 15 گرم در لیتر) و اجسام محلول و درون سلولی داخل سلولی (بازده تا 10 گرم در لیتر) تخصص داریم. ما همچنین پلت فرم تخمیر BSL-2 را برای تولید واکسن های باکتریایی داریم. ما در بهبود فرآیندها، افزایش بازده محصول و کاهش هزینه های تولید متخصص هستیم. ما با یک تیم فناوری موثر، تحویل به موقع و با کیفیت پروژه را تضمین می کنیم و محصولات شما را سریعتر به بازار عرضه می کنیم.
Yaohai Bio-Pharma، 10 تولیدکننده برتر محصولات بیولوژیکی، متخصص تخمیر میکروبی است. ما یک مرکز پیشرفته ایجاد کردهایم که مجهز به امکانات مدرن و قابلیتهای قوی تولید RD است. ما پنج خط تولید مواد دارویی داریم که با الزامات GMP برای تخمیر و خالص سازی میکروبی مطابقت دارند، و همچنین دو خط پایان پرکننده که برای کارتریج ها، ویال ها و همچنین سرنگ های از پیش پر شده خودکار هستند. مقیاس های تخمیر موجود از 100 لیتر تا 500 لیتر، 1000 لیتر و 2000 لیتر متغیر است. تولید PTH نوترکیب برای vias 1 تا 25 میلی لیتر است، در حالی که مشخصات سرنگ های از پیش پر شده و پر کردن کارتریج 1 تا 3 میلی لیتر را پوشش می دهد. کارگاه تولید ما با cGMP سازگار است و عرضه ثابت نمونه های بالینی و همچنین اقلام تجاری را تضمین می کند. کارخانه ما مولکول های بزرگی تولید می کند که به کره زمین ارسال می شود.
Yaohai BioPharma 10 CDMO میکروبی برتر است که مدیریت کیفیت و مسائل نظارتی را در خود جای داده است. ما یک سیستم مدیریت کیفیت جامد را توسعه داده ایم که با استانداردها و مقررات GMP فعلی در سطح جهانی مطابقت دارد. تیم نظارتی ما درک عمیقی از چارچوب های نظارتی در سراسر جهان دارد. این به ما امکان می دهد پرتاب های بیولوژیکی را تسریع کنیم. ما فرآیندهای تولید قابل ردیابی و همچنین محصولات با کیفیت بالا و مطابق با دستورالعمل های FDA ایالات متحده و EMA اتحادیه اروپا را تضمین می کنیم. تولید PTH نوترکیب و NMPA چین نیز راضی هستند. Yaohai BioPharma با موفقیت یک ممیزی در محل توسط یک فرد واجد شرایط اتحادیه اروپا (QP) برای بررسی سیستم GMP و امکانات تولید ما انجام داد. ما همچنین اولین ممیزی های گواهینامه سیستم مدیریت کیفیت ISO9001 و سیستم مدیریت زیست محیطی ISO14001 را انجام داده ایم.