ช่วยให้คุณเข้าใจ CMO, CDMO และ CMO ได้อย่างรวดเร็ว
ในอุตสาหกรรมเภสัชภัณฑ์และชีวเทคโนโลยี การเลือกพันธมิตรที่เหมาะสมสำหรับการพัฒนายาและการผลิตเป็นสิ่งสำคัญ แม้ว่าองค์กรวิจัยตามสัญญา (CRO) และองค์กรผลิตตามสัญญา (CMO) จะให้บริการที่มีคุณค่า แต่องค์กรพัฒนาและผลิตตามสัญญา (CDMO) มีแนวทางที่บูรณาการมากขึ้นซึ่งสามารถปรับปรุงกระบวนการทั้งหมดได้
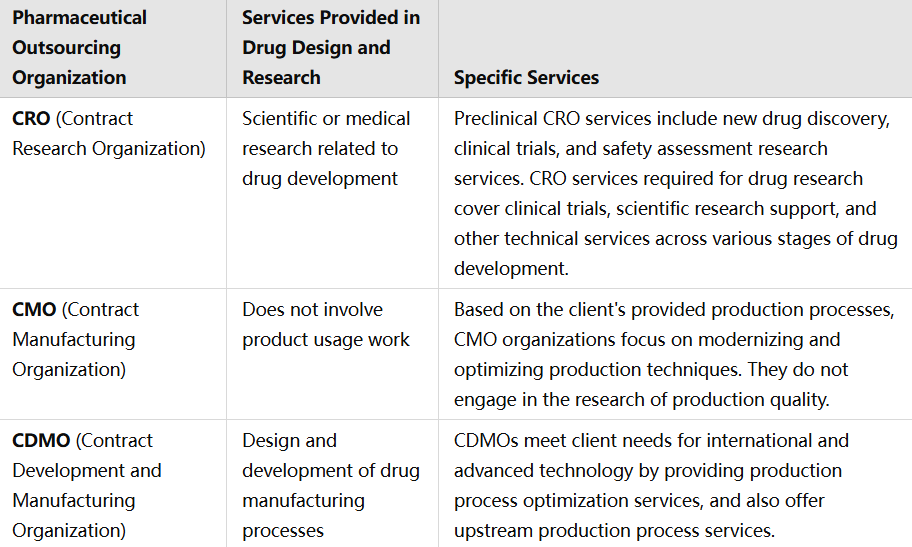
ทำความเข้าใจเกี่ยวกับ CRO, CMO และ CDMO:
องค์กรวิจัยตามสัญญา (CROs): CRO เชี่ยวชาญในระยะการวิจัย โดยให้บริการเช่น การจัดการการทดลองทางคลินิก การวิจัยก่อนคลินิก การจัดการข้อมูล และงานด้านกฎระเบียบ พวกเขาเน้นที่ระยะเริ่มต้นของการพัฒนายาเป็นหลัก
องค์กรผลิตตามสัญญา (CMO): CMO เน้นที่ด้านการผลิต โดยรับผิดชอบการผลิตขนาดใหญ่เมื่อยาได้รับการพัฒนาแล้ว บริการของพวกเขารวมถึงการผลิตเชิงพาณิชย์ การแพ็คเกจ และการทดสอบควบคุมคุณภาพ
องค์กรพัฒนาและผลิตตามสัญญา (CDMO): CDMO ให้บริการแบบครบวงจร ครอบคลุมทั้งการพัฒนาและผลิต พวกเขาให้บริการตั้งแต่การพัฒนาสูตร การปรับปรุงกระบวนการ ไปจนถึงการผลิตวัสดุสำหรับการทดลองทางคลินิกและการผลิตเชิงพาณิชย์ การใช้วิธีการแบบบูรณาการนี้ลดความจำเป็นในการมีหุ้นส่วนหลายรายและทำให้กระบวนการพัฒนาเรียบง่ายขึ้น
ความแตกต่างของบริการใน CRO, CMO, CDMO:
ความสัมพันธ์ระหว่าง CDMO และผลิตภัณฑ์สำหรับขาย
บริการของ CDMO เป็นองค์ประกอบสำคัญในการส่งมอบผลิตภัณฑ์ปลายทาง การพัฒนายาเป็นกระบวนการที่มีความเสี่ยงสูง ลงทุนสูง และใช้เวลานาน เพื่อลดต้นทุนและเพิ่มประสิทธิภาพ บริษัทยาจึง🔍เอาท์ซอร์สการพัฒนากระบวนการยาและการผลิตให้กับ CDMOs บริการเหล่านี้มักจะรวมถึงการออกแบบกระบวนการ การขยายขนาด การยืนยันโครงสร้าง การศึกษาเสถียรภาพ การวิเคราะห์สารปนเปื้อน และการผลิตตามคำสั่ง เมื่อเสร็จสิ้นแล้ว สารกลางหรือ APIs จะถูกส่งมอบให้กับลูกค้า
- บริการของ CDMO ช่วยให้มั่นใจได้ว่ามีการพัฒนาสารกลางหรือ APIs ที่สามารถนำไปใช้เชิงพาณิชย์ได้อย่างประสบความสำเร็จ
- บริการ CDMO เป็นสิ่งสำคัญสำหรับการตอบสนองความต้องการเฉพาะของผลิตภัณฑ์เภสัชกรรมที่ปรับแต่งได้
- ลูกค้าปลายทางเข้าร่วมในการตรวจสอบคุณภาพเพื่อให้มั่นใจในความปลอดภัย ประสิทธิภาพ และคุณภาพ
- CDMO สอดคล้องกับมาตรฐานของอุตสาหกรรม ซึ่งช่วยให้มีบริการและการจัดส่งที่สม่ำเสมอ
ด้วยความพยายามอย่างต่อเนื่องเป็นเวลาหลายปี Yaohai Bio-Pharma ได้สร้างแพลตฟอร์มบริการแบบครบวงจร CRO/CDMO/MAH ระดับแนวหน้าในอุตสาหกรรม จนถึงปัจจุบัน บริษัทได้ส่งมอบโครงการมากกว่า 200 โครงการแล้ว รวมถึงการทดลองทางคลินิกระยะที่สาม 3 โครงการ การทดลองทางคลินิกระยะที่สอง 4 โครงการ การทดลองทางคลินิก IND และระยะที่หนึ่งหลายโครงการ
ในจำนวนนี้ มี 7 โครงการที่ยื่นเอกสารในทั้งสหรัฐอเมริกาและจีน และ 2 โครงการที่ลงทะเบียนในออสเตรเลีย โครงการเหล่านี้ครอบคลุมชีวเวชภัณฑ์หลักและคำแนะนำในการบำบัดหลากหลายประเภท เพื่อให้ตรงตามข้อกำหนดของการยื่นเอกสารกำกับดูแลในหลายภูมิภาคทั่วโลก
เราได้ดำเนินการค้นหาพันธมิตรระดับโลกทั้งสถาบันและบุคคลอย่างต่อเนื่อง เราเสนอค่าตอบแทนที่แข่งขันได้มากที่สุดในอุตสาหกรรม หากท่านมีคำถามใด ๆ กรุณาติดต่อเราได้ที่ BD@yaohaibio.cn
ข่าวร้อน
-
ยาโอไฮ ไบโอ-ฟาร์มา ผ่านการตรวจสอบของยุโรป QP และได้รับการรับรอง ISO สามมาตรฐาน
2024-05-08
-
BiotechGate, ออนไลน์
2024-05-13
-
งานประชุมวัคซีนโลก 2024 วอชิงตัน
2024-04-01
-
CPHI 北美 2024
2024-05-07
-
BIO International Convention 2024
2024-06-03
-
FCE COSMETIQUE
2024-06-04
-
CPHI Milan 2024
2024-10-08

 TH
TH
 EN
EN AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN

