Tilpasning, Effektivitet & Kostnadseffektivitet
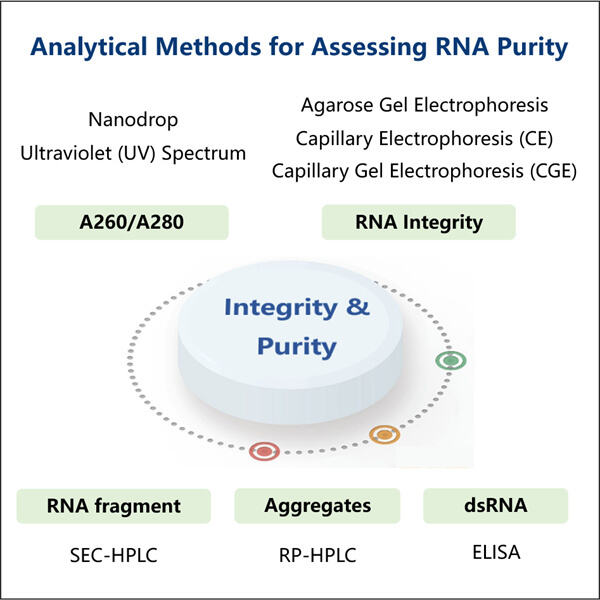
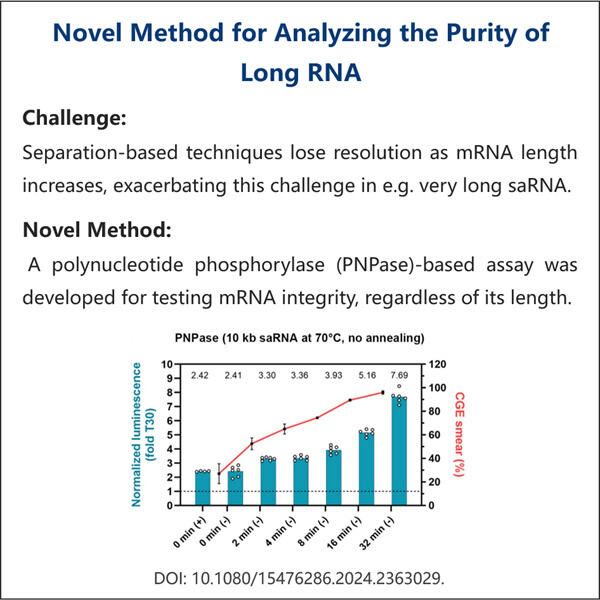
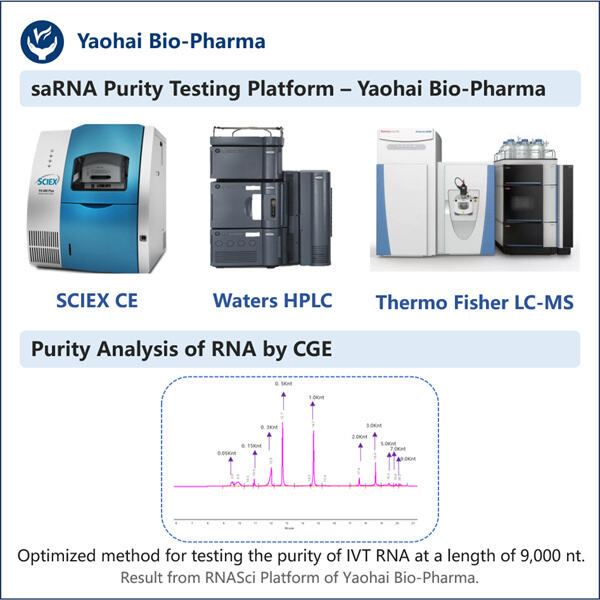
Yaohai Bio-Pharma har erfaring inden for udvikling af mikrobielt-baserede biologiske produkter. Vi tilbyder tilpasset RD samt produktionsløsninger, samtidig med at vi sikrer, at der ikke er nogen risici. Vi har arbejdet med forskellige modaliteter såsom subenhedsbaserede rekombinante vacciner, saRNA Renhedstestning, cytokiner, vækstfaktorer, enkeltområdesantikörper, enzymmer, plasmid DNA, mRNA og andre. Vi er ekspert i en række mikroorganismer, herunder gær ekstracellulær og intracellulær sekretion (opnåelser op til 15g/L) samt bakterielle intracellulære løselige og inklusionslegemer (opnåelser op til 10g/L). Vi har også udviklet BSL-2 fermentationsplatformen til at skabe bakteriebaserede vacciner. Vi har en god ry for at forbedre produktionprocesser, hvilket øger udbyttet og reducerer omkostningerne. Med et højtydende teknisk team sikrer vi tidsmæssig og kvalitetsfuld projektudlevering og bringer dine produkter hurtigere på markedet.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN