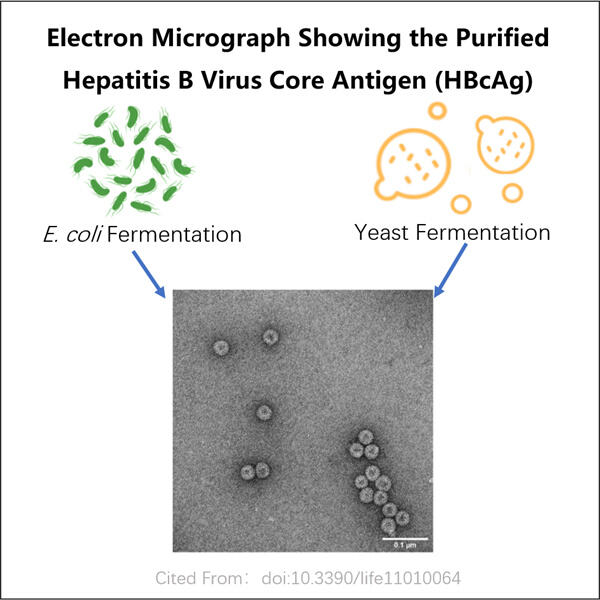
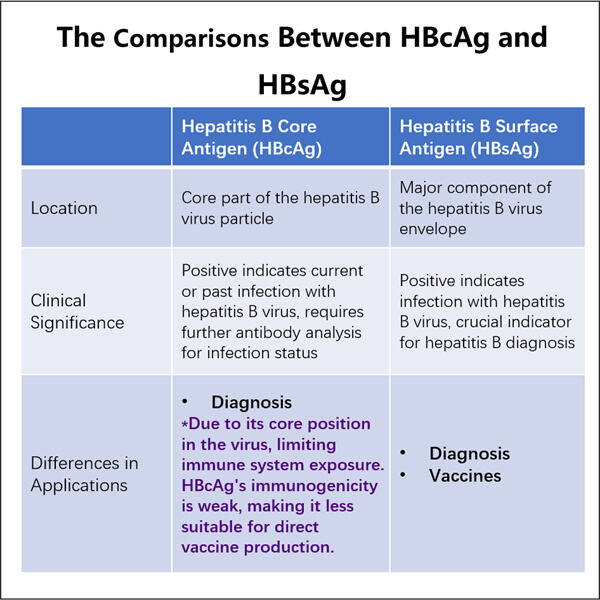
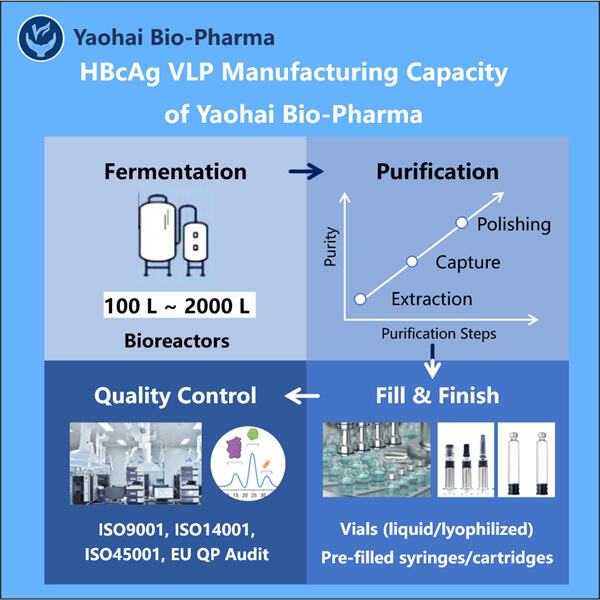
L'epatite B è un'attitudine che milioni di persone soffrono a livello globale. È un'infezione epatica causata dal virus dell'epatite C, che può anche portare a cancro al fegato se non trattata. Il fegato è un organo vitale che aiuta nella digestione e filtra materiali dannosi dal sangue. L'epatite B può causare gravi disturbi, uno dei motivi per cui è molto critico scoprire come possa essere prevenuta e curata. La società Yaohai Processo di Fermentazione e Purificazione VLP sta correndo per produrre vaccini e trattamenti affinché tutti possano tenere l'epatite B sotto controllo.
Attualmente, l'epatite B dispone di vaccini più efficaci, ma non vengono presentati come il modo ideale per farsi vaccinare. Anche se una persona è completamente vaccinata, ci sono comunque casi in cui si può ammalare e contrarre il virus. Il problema è che l'epatite B può essere mortale, soprattutto per chi ha il corpo debole, come i neonati e le persone anziane. Per questo motivo si intende sviluppare vaccini migliori che aiutino le persone a proteggersi dalle infezioni e le difendano da questo virus pericoloso.

 IT
IT
 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN