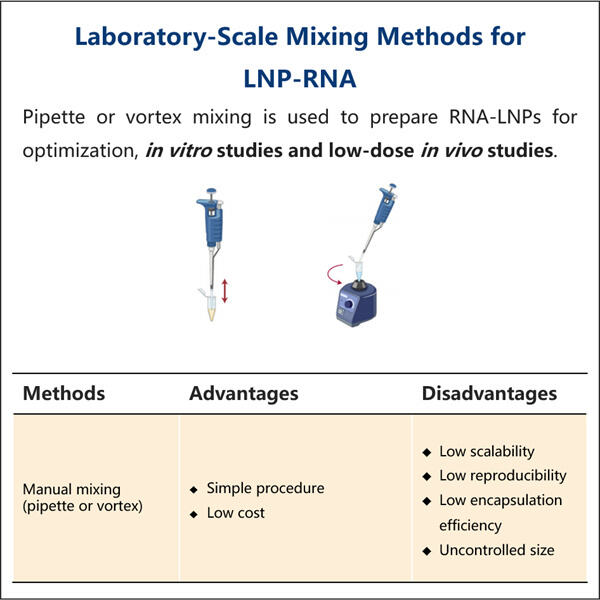
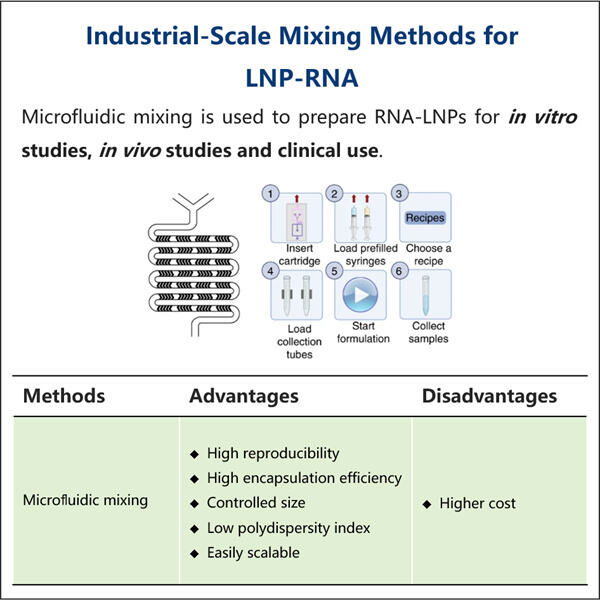
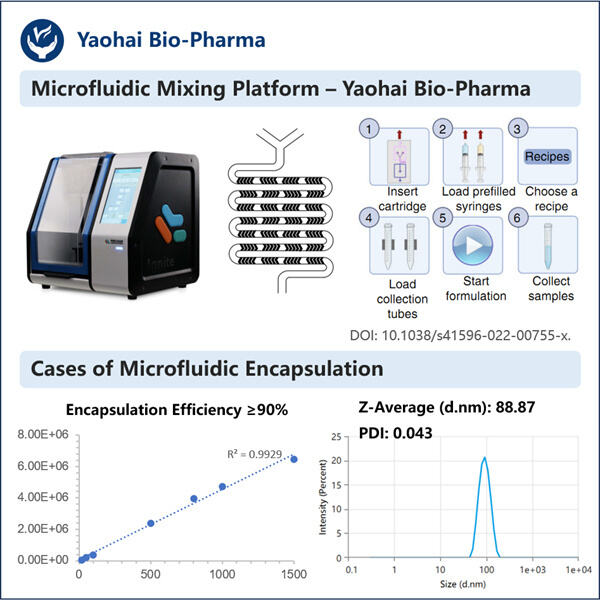
LNP kapseldamise võrdlemine teiste ravimite kohaletoimetamise meetoditega
LNP Encapsulationil on ka eeliseid, kui see muudab ravimi jõudmise meie rakkudesse palju lihtsamaks. Meie rakke ümbritsevad paksud seinad või membraanid, millest ravimitel võib olla raske tungida. Kujutage ette, et soovite siseneda majja, mille uks on lukus. LNP Encapsulation hiilib ravimi nendest seintest mööda – nagu oleks ukse võti –, et toimetada see rakkudesse, mis seda vajavad. Sillutab teed ravimite tõhusamaks kohaletoimetamiseks.
Lisaks võib teiste ravimite manustamisviiside mõju võtta kauem aega. Näiteks võib teatud ravimite kehas lahustumine või kogu kehas levimine võtta aega. LNP kapseldamine võimaldab ravimil kergemini rakkudesse siseneda, mille tulemuseks on ravimi kiire toime organismis. Tore, kui keegi on haige ja vajab kiiret abi.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 EI
EI
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN