Semaglutide
Description of Semaglutide
Semaglutide is a recombinant human glucagon-like peptide-1 (GLP-1) analogue, belonging to the class of GLP-1 receptor agonists (or GLP-1 RAs). Novo Nordisk developed three formulations of semaglutide already available in the market: Ozempic and Wegovy (injection formulation) and Rybelsus (oral tablet).
Ozempic and Rybelsus are indicated for glycemic control in type 2 diabetes mellitus patients, while Wegovy is for weight management in obesity or overweight patients.
Synonyms
Semaglutide, Ozempic (injectable formulation for glycemic control), Wegovy (injectable formulation for weight management), Rybelsus (oral semaglutide), NN9535, NNC0113-0217, CAS 910463-68-2, UNII-53AXN4NNHX
Amino Acid Sequence of Semaglutide
Semaglutide is a well-designed long-acting GLP-1 (7-37) analogue based on liraglutide. Semaglutide differs from liraglutide in two sites. Position 8 is replaced by Aib(U)——a modification that significantly protects it from enzymatic degradation by the dipeptidyl-peptidase 4 (DPP-4). In addition, the modified fatty acid chain and two PEG2 intermediates are optimal for prolonged efficacy both in vitro and in vivo.
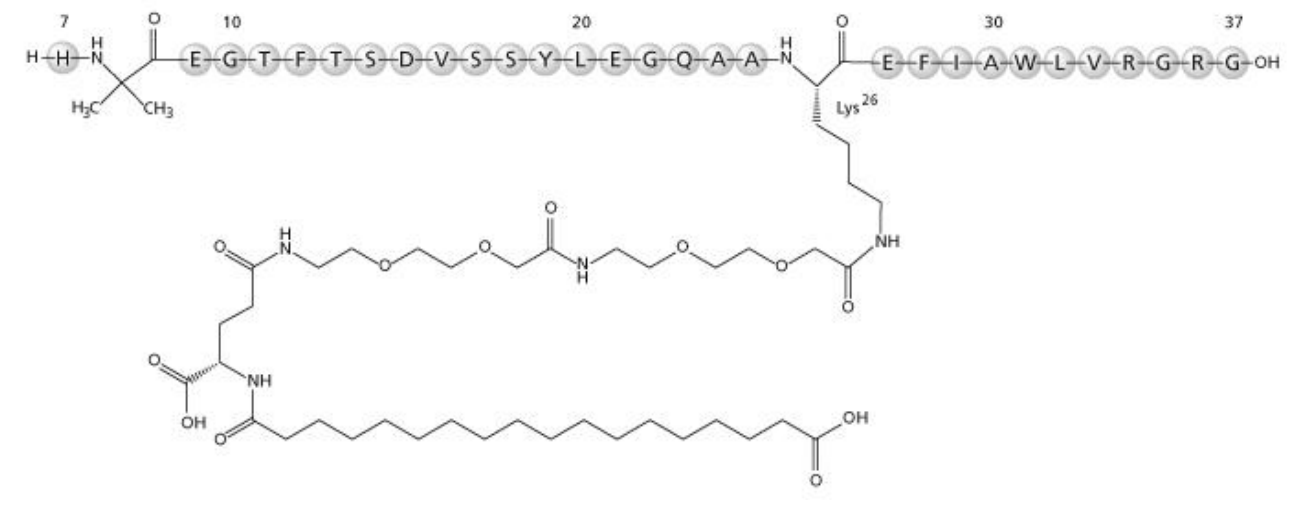
Fig 1. Structure formula of Semaglutide
Formulation of Semaglutide
Expression system
Yeast
Manufacturing Process of Semaglutide
Drug Substance Manufacturing
Yeast fermentation and culture broth harvesting
Purification to produce pure peptide backbone (also called GLP-1(9-37) or semaglutide precursor)
Peptide modification and purification to form active substance
Drug Product Manufacturing
Aseptic Fill-Finish: Preparation of formulation and filling it into cartridges, followed by finishing

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN