The design concept of microbial vector (live bacterial vector) vaccines is to modify attenuated pathogens or symbiotic bacteria based on genetic engineering technology to deliver target antigens and activate the body's immune response. The greatest advantage of live bacterial vectors is that they can stimulate a wide range of humoral immunity and cellular immunity. The development direction of microbial vector vaccines includes the prevention of infectious diseases and the treatment of tumors.
Yaohai Bio-Pharma has more than ten years of microbial CDMO experience. We have established a GMP workshop with Biosafety Level 1 (BSL-1) and Biosafety Level 2 (BSL-2), and launched a one-stop solution for microbial vector vaccine CDMO, covering from microbial strain development to GMP production. Based on the customized needs of customers, we provide customers with bacterial bodies (DS, API) or live bacterial drug products (DP) that meet quality standards, as well as GMP production records and test reports.
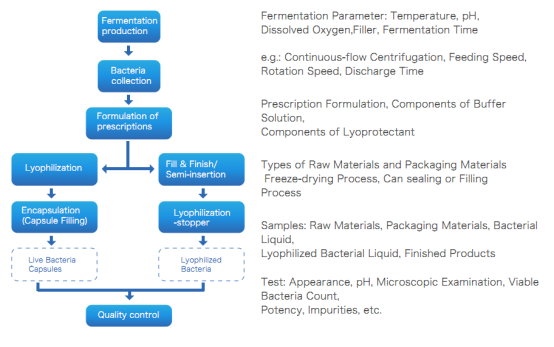
Microbial Vector Vaccine Production Process
Microbial vector vaccines are also live bacterial preparations, and their preparation process is the same as the [attenuated live vaccine preparation process.
Deliverables
|
Grade
|
Deliverables
|
Specification
|
Applications
|
|
GMP, BSL-1/BSL-2
|
Bacterial Cell (DS, drug substance)
|
Bacterial Suspension
|
Investigational new drug (IND),
Clinical trial authorisation (CTA),
Clinical trial supply,
Biologic license application (BLA),
Commercial supply
|
|
Lyophilized Bacterial Cell
|
|
Live Bacterial (DP, drug product)
|
Vials (liquid)
|
|
Vials (lyophilized)
|
|
Other dosage forms
|

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN