Conjugation of the target antigen with carrier proteins is also a development strategy for vaccines, such as conjugate vaccines. Binding with carrier proteins can enhance the immunogenicity of vaccines. Currently, approved carrier proteins on the market include diphtheria toxoid (DT), non-toxic mutant of diphtheria toxin CRM197, tetanus toxoid (TT), etc.
With a powerful process development platform, biosafety level BSL-1 and BSL-2 workshops, and a GMP quality system, Yaohai Bio-Pharma can provide customers with a one-stop solution from microbial strain development to GMP production of carrier proteins.
We deliver gram-to-kilogram-scale carrier proteins that meet quality standards, as well as GMP production records and testing reports to our customers.
Approved conjugated vaccines include the following carrier proteins (non-recombinant):
|
Protein Types
|
Carrier Proteins
|
Strain Types
|
Platform
|
|
Carrier protein, extracted
|
Diphtheria toxin non-toxic mutants CRM197
|
Corynebacterium diphtheriae
|
Microbial fermentation systems
Centrifugation and homogenization equipment
High/low-pressure chromatography systems
Conjugate reaction tank
Biosafety Level: BSL-2
GMP quality system
|
|
Diphtheria toxoid (DT)
|
Corynebacterium diphtheriae
|
|
Tetanus toxoid (TT)
|
Clostridium tetani
|
|
Meningococcal outer membrane protein complex (OMPC)
|
Neisseria meningitidis
|
|
Other carrier proteins from microbial sources (BSL-1, BSL-2)
|
|
Related services: Recombinant Carrier Protein CDMO Solutions.
|
Carrier Protein Drug Product Process
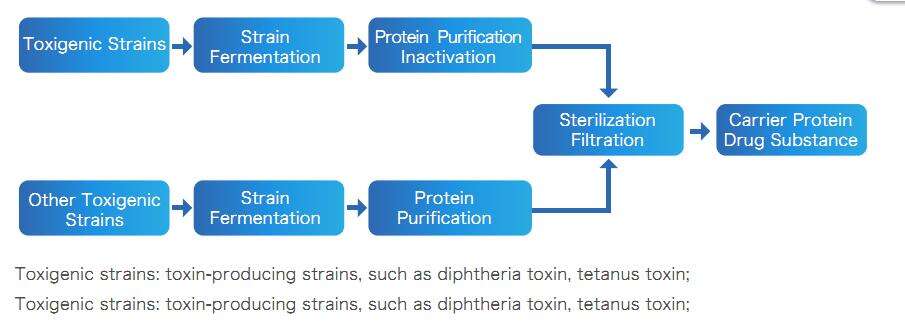
Deliverable
| Grade |
Deliverables |
Specification |
Applications |
| GMP, BSL-1/BSL-2 |
Intermediate substance |
Bacteria derived carried protein |
Conjugated vaccine production materials |
| Vaccine finished product |
Lyophilized powder |
Investigational new drug (IND),
Clinical trial authorisation (CTA),
Clinical trial supply,
Biologic license application (BLA),
Commercial supply
|

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN


