The attenuated live vaccine is a kind of vaccine development strategy, which is usually natural attenuated strains, artificial pass-through screening, or directional modification of attenuated strains. These vaccines maintain their immunogenicity and stimulate immune responses in the body, effectively preventing diseases. Live attenuated vaccines have been successfully utilized for the prevention of viral or bacterial infections, including human typhoid Salmonella live vaccine, attenuated cholera vaccine, as well as veterinary vaccines like Bordetella bovis vaccine, Bartonella multocida porcine pleuropneumonia live vaccine, and piglet paratyphoid live vaccine.
Yaohai Bio-Pharma has over ten years of experience as a microbial CDMO, providing Contract Development and Manufacturing services for live attenuated bacterial vaccines.
Our Biosafety Level 2 (BSL-2) operational area ensures the highest level of safety during microbial strain development, GMP drug production, and aseptic Fill & Finish. We offer customized solutions tailored to the unique requirements of our clients, delivering bacterial cell banks (Drug Substance, API) or live bacterial drug products that meet the highest quality standards. Our GMP production records and testing reports provide our clients with complete transparency and confidence in our services.
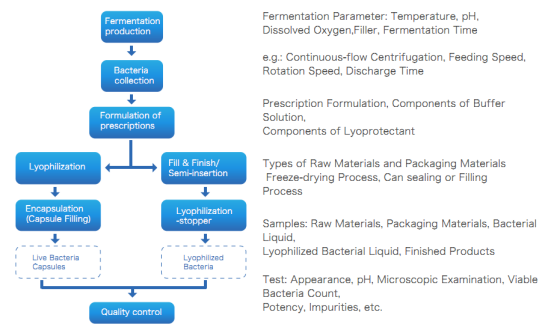
Production Process
Deliverables
|
Grade
|
Deliverables
|
Specification
|
Applications
|
|
GMP, BSL-1/BSL-2
|
Bacterial Cell (DS, drug substance)
|
Bacterial Suspension
|
Investigational new drug (IND),
Clinical trial authorisation (CTA),
Clinical trial supply,
Biologic license application (BLA),
Commercial supply
|
|
Lyophilized Bacterial Cell
|
|
Live Bacterial (DP, drug product)
|
Vials (liquid)
|
|
Vials (lyophilized)
|
|
Other dosage forms
|

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN