The Significance of Formulation Development
Biological drugs, such as recombinant proteins or peptides, are less stable than small molecule drugs. If a drug cannot be delivered in a stable form, it may not even go beyond first-in-human (FIH) studies.
Therefore, formulation development is one of the most critical aspects in the biological lifecycle for ensuring drug quality, efficiency, and stability during manufacturing, transport, long-term storage, and administration.
Keywords: Biopharmaceutical formulation development and optimization, biologics dosage form, drug formulation composition, pre-formulation studies, formulation research, formulation screening
Application: Biopharmaceutical industry, human medicine, animal medicine, vaccine, recombinant large molecule biologics, biologics, biological reagent
Formulation Development Services of Yaohai Bio-Pharma
Liquid and lyophilized (freeze-dried) formulations currently represent the most common administration routes for biologics.
Yaohai Bio-Pharma specializes in developing liquid Drug Substance (DS) or Drug Product (DP) as well as lyophilized DP in vial or prefilled syringes for different administration routes, including intravenous (IV), subcutaneous (SC), intravitreal (IVT), and inhalation (INH).
We apply One-Time-A-Factor (OTAF) or Design-of-experiments (DoE) to phase-appropriate formulation development and optimization, including the following steps:
- Pre-formulation testing of protein physicochemical properties and stability
- Liquid Drug Substance (DS) formulation screening and optimization
- Liquid Drug Product (DP) formulation screening and optimization
- Lyophilized Drug Product (DP) formulation screening and optimization
- Fill-Finish process and lyophilization cycle development
- Standard real-time and accelerated stability, and acute stress studies
Service Details
| Service Details |
Unit Operations |
Our Focus |
| Pre-formulation testing |
Physicochemical properties Stability testing |
Decide upon a suitable formulation (e.g., liquid, lyophilized) for early or late clinical trials |
| Liquid DS or DP formulation |
High-throughput liquid formulation screening |
Buffer compositions, pH, Ionic strength, stabilizers, surfactants, excipients, adjuvants, etc. |
| Lyophilized DP formulation |
High-throughput lyophilized formulation screening |
Lyoprotectant (e.g., sucrose, trehalose), buffer system, excipients, etc. |
| Process development for liquid DP |
Adjuvant preparation and sterilization technique - optional |
Stability studies of adjuvant formulation |
| DS dilution and DP preparation |
Dose strength, pumping study, stirring speed, shear forces |
| Fill and Finish |
Fill volume, mixing study, shear forces |
| Lyophilization process development |
Lyophilization cycle development |
Lyophilized DP quality |
| Quality Testing |
Purity, integrity, solubility, viscosity, activity, and aggregation, etc. |
The effect of formulation compositions and processes on DS/DP quality |
| Stability studies |
Case Study
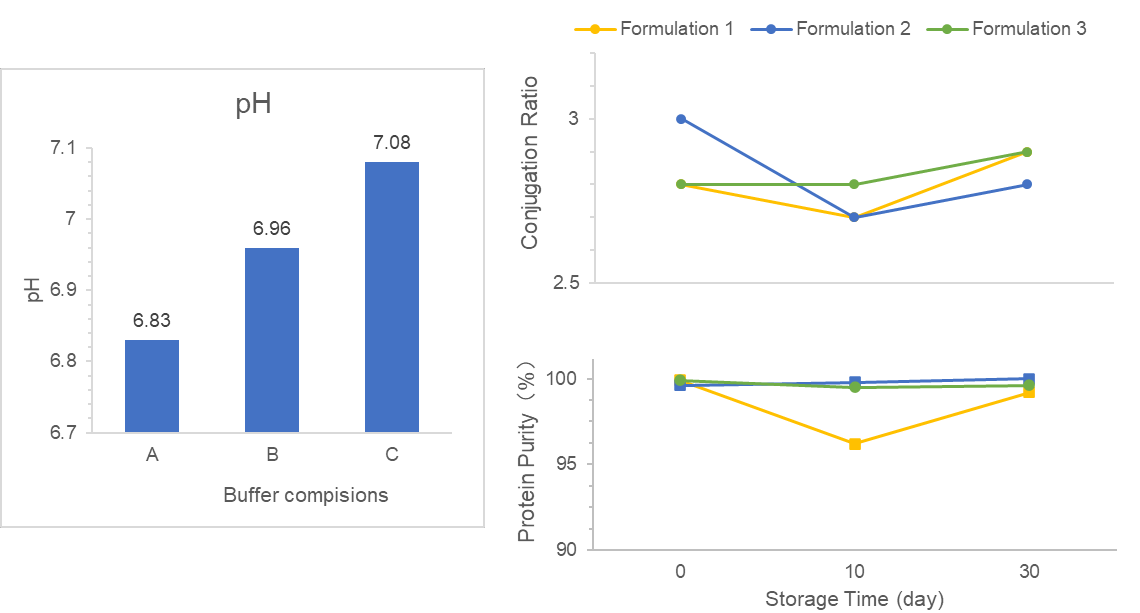
We are commissioned to screen DS/DP formulation and design DS/DP process for a VLP-conjugate vaccine.
Firstly, we screened suitable buffers in DS formulation, meeting desired stability, bioavailability, and clinical safety requirements. Secondly, we optimized several factors in adjuvant-based DP formulation to improve antigen adsorption levels. Additionally, we focused on DS/DP quality and developed a stable manufacturing process.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN


