| Process |
Optional Service |
Service Details |
Delivery Period (workday) |
Deliverable |
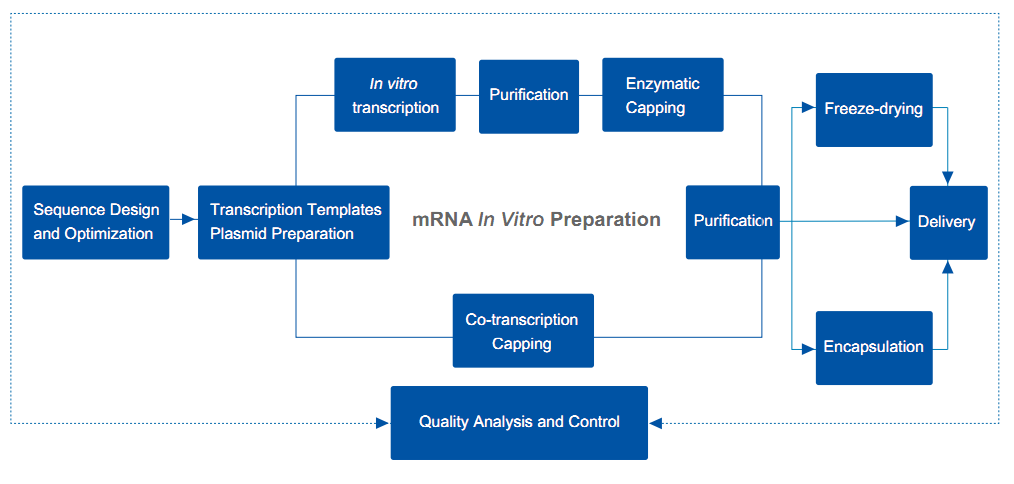
| mRNA Sequence Design and Optimization |
Design and optimization of coding sequences |
CDS sequence design and codon optimization |
1-3 |
Sequence file |
| Design and optimization of non-coding sequences |
Design and optimization of UTR, polyA sequences |
| Transcription Template Plasmid Preparation |
Recombinant plasmid preparation |
Gene synthesis |
7-10 |
| Plasmid amplification and extraction |
4 |
| Plasmid linearization and purification |
| mRNA In Vitro Transcription |
Co-transcriptional capping (one-step method) |
In vitro transcription (IVT) / Cap analog |
1-2 |
N/A |
| Nucleotide modifications (UTP/CTP modifications) |
| DNA template removal (DNase I) |
| Enzymatic capping (two-step process) |
IVT |
2-3 |
| Nucleotide modifications (UTP/CTP modifications) |
| DNA template removal (DNase I) |
| mRNA purification (lithium chloride/magnetic beads) |
| Enzymatic capping |
| mRNA Purification |
Conventional purification scheme |
Lithium chloride precipitation |
1 |
mRNA drug substance |
| Magnetic bead purification |
| Chromatography column purification scheme |
Combination of multiple chromatography methods |
1-2 |
| Buffer exchange |
Ultrafiltration |
1 |
| mRNA Lyophilization |
Lyophilization |
Pre-freezing |
2-3 |
mRNA lyophilized powder |
| Primary drying (sublimation) |
| Secondary drying (desorption) |
| mRNAEncapsulation |
LNP encapsulation |
LNP encapsulation |
2-3 |
mRNA-LNPDrug product |
| Concentration and buffer exchange |
| mRNA Quality Analysis |
mRNA drug substance/ lyophilized powder |
Concentration, purity |
1 |
Test Report |
| Integrity, capping efficiency, polyA tail distribution |
2-5 |
| mRNA-LNP drug product |
Encapsulation efficiency |
1 |
| Particle size and distribution |
| Surface charge |
| mRNA Expression Validation |
293T cell evaluation |
Cell plating |
4 |
| Transient transfection of cells |
| Fluorescence signal observation |
1-3 |
| Western blot (WB)/ Enzyme-linked immunosorbent assay (ELISA) |

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN