The basis of encapsulation is the design and development of the delivery system. A well-designed delivery system allows mRNA molecules to enter the body without being degraded by RNase, and then be effectively delivered to the target site, cross the cell membrane and be released intracellularly. Lipid nanoparticles (LNPs) are the optimal delivery systems available, with advantages in terms of encapsulation, delivery, and safety compared to other delivery systems. LNPs with nucleic acid fragments are easily swallowed into cells and form intracellular bodies. Once inside the cell, the acidic environment of the intracellular body protonates and positively charges the head of the ionized lipid, which fuses with the inner membrane of the intracellular body and releases the target nucleic acid into the cell for action.
Yaohai Bio-Pharma mRNA service continues to improve, and now can provide mRNA-LNP encapsulation service, optimize relevant critical process parameters, and improve the consistency and reproducibility of mRNA drug production.
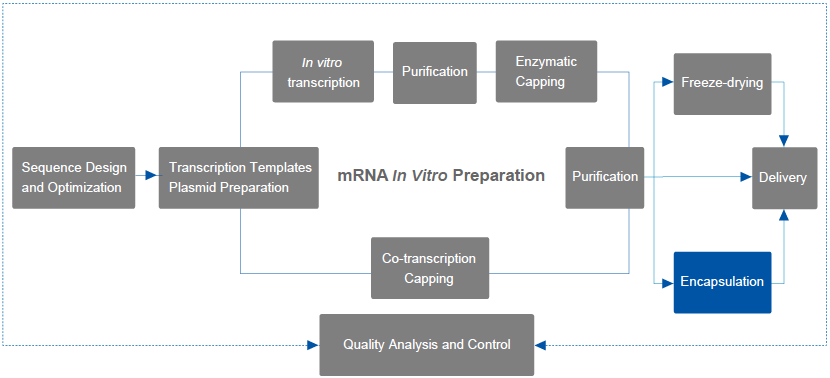
Services Details
| Service | Service Details | Delivery Period (Workday) | Deliverables |
| mRNA-LNP encapsulation | Preparation of water phase (mRNA) and lipid phase | 2 | mRNA-LNP drug product (DP) |
| Microfluidic device mixing |
| Ultrafiltration concentration | 1 |
| Sterilizing filtration |
| mRNA-LNP quality control | Encapsulation efficiency | 1 | Test Report |
| Particle size and distribution detection |
| Surface charge detection |
| mRNA-LNP expression validation | 5-7 |
Our Features
Fast synthesis speed, high R&D efficiency and pre-optimized solutions available.
mRNA-LNP encapsulation rate can reach more than 90%.
By changing the fluid injection rate and ratio, the LNP particle size is controlled between 80 and 100nm, and the particle size distribution is uniform (PDI<0.10).
mRNA-LNP is validated by in vitro cell expression and can express the target protein efficiently.
Case study
With the help of Yaohai Bio-Pharma's microfluidic encapsulation platform, we optimized the mRNA encapsulation conditions, including the ratio of each component of LNP, the ratio of aqueous phase to organic phase, and the flow rate of each microfluidic phase. The particle size and particle size distribution of the finished mRNA-LNP were then detected.
Experimental results show that through preparation formulation and process optimization, we control the LNP particle size to 80~100nm, and the dispersion coefficient (PDI) is 0.043, indicating that the LNP size distribution is uniform.
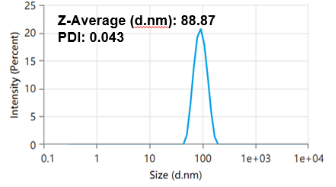
Analysis of a mRNA-LNP sample

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN