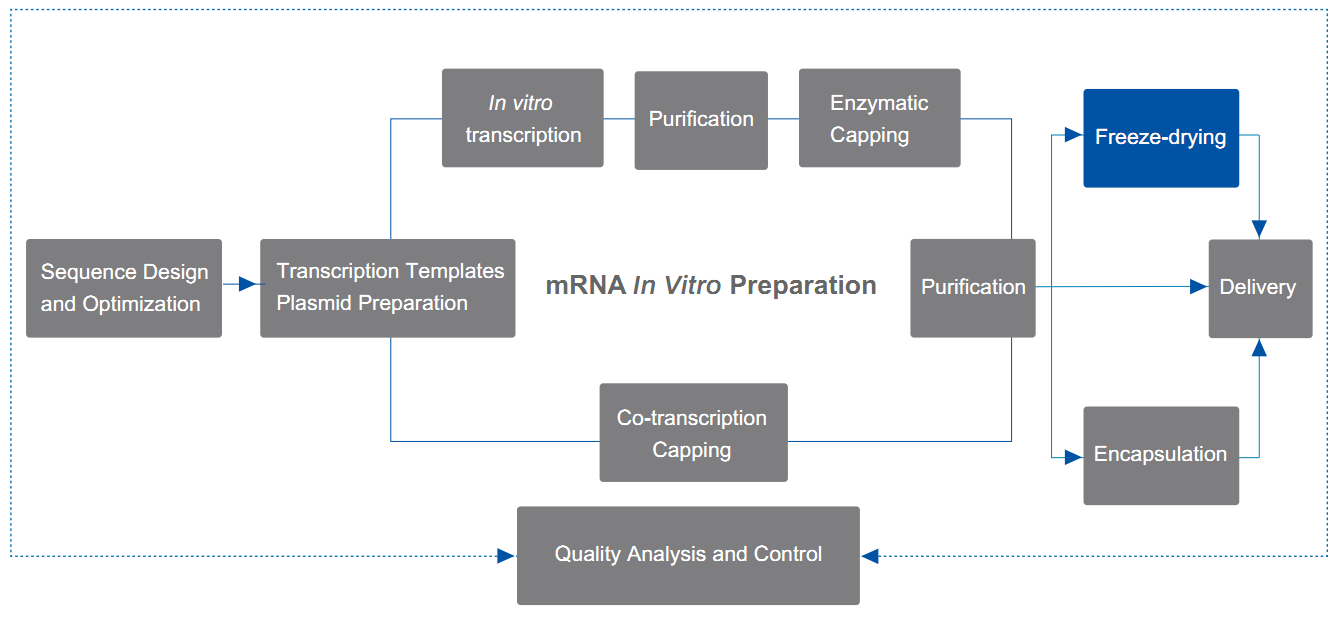
In order to improve the stability of mRNA and avoid degradation in storage and transportation, Yaohai Bio-Pharma can provide mRNA lyophilization (freeze-drying) services for customers to freeze-dry the mRNA drug substance (DS) and store or transport it in the form of lyophilized powder.
- Dispensing
- Pre-freezing
- Primary drying
- Secondary drying
Services Details
Process |
Optional Service |
Service Details |
Delivery Period (Day) |
Deliverables |
mRNA lyophilization |
Filling |
Filling |
2-3 |
mRNA lyophilized powder |
| Lyophilization |
Pre-freezing |
| Primary drying (sublimation) |
| Secondary drying (desorption) |
mRNA quality control |
Solubility of lyophilized powder |
Resuspension |
- |
Test Report |
| Appearance inspection |
- |
| Concentration measurement |
Ultraviolet spectrophotometry (UV) |
0.5 |
| Integrity and purity testing |
Agarose gel electrophoresis (AGE) |
| Capillary electrophoresis (CE)-optional |
1 |
Our Features
- Mature Lyophilization Process
Lyophilization has no effect on mRNA integrity.
- Homogeneous Quality Properties
mRNA samples before and after lyophilization can successfully express the target protein.
mRNA lyophilized powder is easy to store and transport.
Case study
Using conventional liposomes, mRNA samples before and after lyophilization are transfected into 293T cells for cellular evaluation. The results show that strong fluorescence signals are observed in the catalog eGFP mRNA sample products before and after lyophilization, indicating that the target protein was efficiently expressed in vitro.
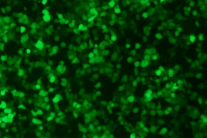
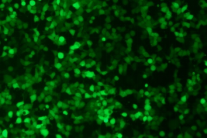
eGFP mRNA samples before (left) and after (right) lyophilization

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN