The Significance of LNP Lipid Composition Analysis
Lipid nanoparticles (LNPs), as effective vehicles to deliver small or large RNA molecules, have successfully entered the clinic. LNP typically contains four lipid components: cationic lipid, PEG lipid, helper lipid, and cholesterol.
LNP formulation development is one of the most critical aspects of the mRNA life cycle for ensuring drug quality, efficiency and stability during manufacturing, transport, long-term storage, administration and delivery.
LNP Formulation Solutions of Yaohai Bio-Pharma
- Linear plasmid process development and optimization
- LNP process development by microfluidics device
- mRNA-LNP drug product (DP) formulation development
Methodology
Quality by Design (QbD)
Design of Experience (DoE)
One Factor at a Time (OFAT)
Service Details
|
Service Details
|
Unit Operations
|
Parameters
|
|
LNP Lipid Components Screening
|
High-throughput formulation screening
|
Lipidtype, molar lipid ratios
|
|
LNP Process Development
|
Microfluidics technology
|
Molar N/P ratios, ratio of aqueous phase to ethanol phase, feed flow rate
|
|
mRNA-LNP Formulation Development
|
Lipid DP formulation screening
|
Buffer compositions, pH, Ionic strength, stabilizers, surfactants, excipients,etc.
|
|
Lyophilized DP formulation screening
|
Lyoprotectant (e.g., sucrose, trehalose),buffer system, excipients,etc.
|
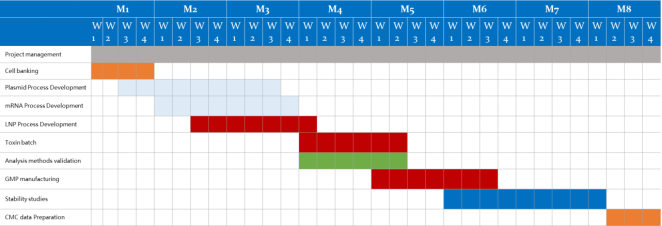
Timeline of mRNA CDMO Solutions

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN