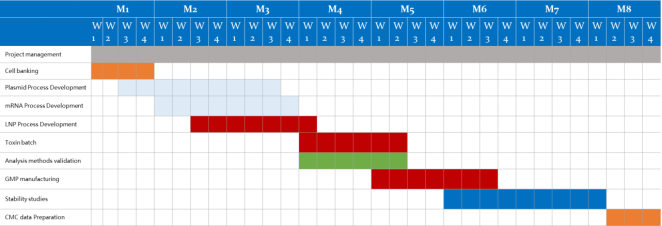
| Service Details |
Unit Operations |
Parameters |
| Single-enzyme Digestion |
Digestion reaction |
Reaction components, volume, rotational speed |
| Linearized DNA Purification |
IEX, Anion or cation exchange chromatography |
Types of chromatography resin/column Buffer composition, Mobile phase composition, pH, Flow rate, binding, washing and elution conditions. |
| RPC, Reversed phase chromatography |
| mRNA Synthesis by IVT |
IVT reaction |
IVT reaction components, volume, rotational speed, IVT time |
| DNA removal |
DNase concentration |
| Enzymatic Capping |
Capping reaction |
Reaction components, volume, rotational speed |
| mRNA Purification |
AC, Affinity chromatography |
Several types of chromatography resin/column (e.g., AC, IEX, RPC, SEC, MMC), Buffer composition, injection volume, Mobile phase composition (adsorption and desorption), pH, flow rate, binding, washing, and elution conditions. |
| IEX, Anion, or cation exchange chromatography |
| SEC, Desalting, and/or size exclusion chromatography |
| RPC, Reversed-phase chromatography |
| MMC, Mixed mode chromatography |
| Tangential flow filtration |
Membrane material and pore size, feed flow rate, transmembrane pressure (TMP), filtrate volume to membrane area ratio |

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN