Description of Insulin Lispro, Recombinant
Insulin lispro is a fast-acting insulin comprising human insulin analog with the engineered amino acid sequence at B chain, with the chemical name of Lys(B28), Pro(B29) human insulin analog.
Formulations based on insulin lispro have been available on the market since 1996 with the brand name Humalog (Lilly); and other available products include Lyumjev (Lilly), and Admelog (Sanofi).
Synonyms
Insulin Lispro (Genetical Recombination), insulin lispro-aabc, Eglucent, Humalog, Humalog Cart, Humalog Miriopen, Humalog Mix, Liprolog, Liumjev, LY-275585, LY-900014, LY-900027, LYS-B28, LYSPRO, Lyumjev, PRO-B29, ルムジェブ, Admelog
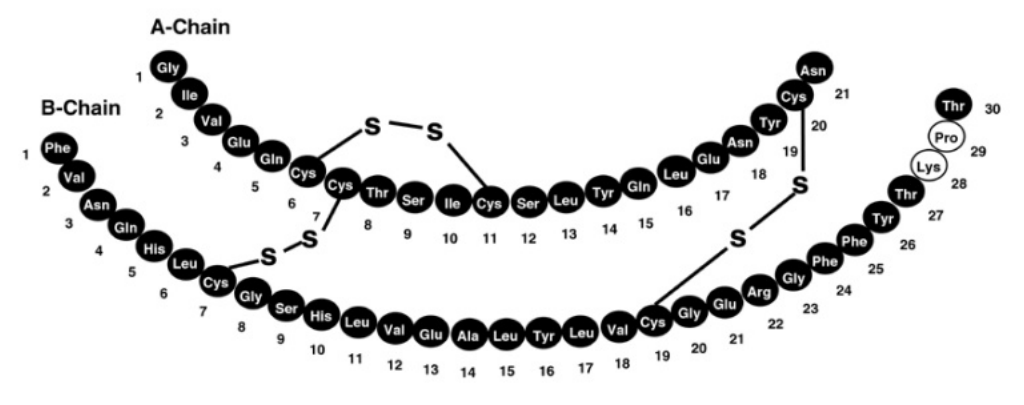
Fig 1. Structure formula of insulin lispro
Expression system of Insulin Lispro
|
Brand Name
|
Active Substance
|
Expression System
|
|
Humalog
|
Insulin lispro
|
Bacteria (Escherichia coli)
|
|
Liprolog
|
Insulin lispro
|
Bacteria (Escherichia coli)
|
|
Lyumjev
|
Insulin lispro
|
Bacteria (Escherichia coli)
|
|
Admelog
|
Insulin lispro
|
Bacteria (Escherichia coli)
|
Formulation of Insulin Lispro
|
Inactive ingredients (Excipients) in Humalog (Vial, Prefilled Cartridge)
Dibasic sodium phosphate, glycerin, metacresol (m-cresol), phenol (trace amounts), zinc oxide, and hydrochloric acid or sodium hydroxide (pH 7.0-7.8).
|
|
Inactive ingredients (Excipients) in Liprolog (Vial, Prefilled Cartridge)
Dibasic sodium phosphate, glycerin, metacresol (m-cresol), zinc oxide.
|
|
Inactive ingredients (Excipients) in Lyumjev (Vial, Prefilled Cartridge)
Glycerol, magnesium chloride hexahydrate, metacresol, sodium citrate dihydrate, treprostinil sodium, zinc oxide, and hydrochloric acid or sodium hydroxide (pH 7.0-7.8).
|
|
Inactive ingredients (Excipients) in Admelog (Vial, Prefilled Cartridge)
Dibasic sodium phosphate, glycerin, metacresol (m-cresol), zinc oxide, and hydrochloric acid or sodium hydroxide (pH 7.0-7.8).
|
Manufacturing Process of Insulin Lispro
Upstream Process
- E. coli strain expanded in a shake flask from a vial of Working Cell Bank (WCB);
- Fermentation: seed fermentation; and induction of expression of insulin lispro by high-density fermentation.
Downstream Process
- Harvesting E. coli cells and inclusion bodies by centrifugation and cell lysis.
- Refolding of inclusion bodies to produce insulin lispro precursor;
- Enzymatic cleavage and purification to obtain pure active substance.
Fill and Finish
- Formulation preparation and aseptic fill-finish in vials or prefilled cartridges.
Yaohai Bio-Pharma Offers One-Stop CDMO Solution for Insulin

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN


