The Significance of Strain Screening
Screening suitable hosts is necessary for biologics CMC development, which determines the quality and consistency of the product and manufacturing process. The direct objective of host screening is to provide a candidate primary cell bank (PCB), with high expression levels and high stability both in terms of passage stability and storage stability. The screening of suitable host is necessary for two main reasons:
- First, to select the microbial host with a high expression level. There are multiple commercially available strains, which are used to express different types of proteins, like toxic proteins, soluble proteins, or eukaryotic proteins.
- Secondly, to screen the host with genetic stability. Genetic stability testing is a key point throughout the production life cycle. It is suggested that high genetic stability is recognized as another key characteristic for suitable strain.
Keywords: microbial strain selection, microbial strain screening, high-throughput screening of bacteria or yeast, high-producing strain screening, highly stable strain screening, genetic stable strain screening Application: human medicine, animal medicine, vaccine, synthetic biology, recombinant large molecule biologics, biological reagent
Strain Screening Services of Yaohai Bio-Pharma
Benefiting from over 10 years of CDMO experience in microbial biologics, we support the development and manufacturing of various large molecules, including recombinant subunit vaccines, virus-like particles (VLP), hormones (insulin, GLP-1, growth hormone), cytokines (Interleukin-2/IL-2, IL-15, IL-21), growth factors(EGF, FGF, NGF), nanobodies/Single-domain antibodies (sdAbs), enzymes, etc.
We have accumulated rich experiences in the selection of various types of plasmid vectors, and microbial hosts, including Bacteria Escherichia coli (E. coli), and yeast Pichia pastoris (P. pastoris), Saccharomyces cerevisiae (S. cerevisiae), Hansenula polymorpha (H. polymorpha). We can find the best expression system for your unique biologics.
We collected various microbial hosts with clear sources and standard CoA, including E.coli expression and yeast expression.
Service Details
|
Services
|
Service Details
|
Minimum Timeline/Day
|
Deliverables
|
|
Strain Construction (E. coli)
|
Plasmid transformation (multi-hosts)
|
5
|
COA of commercial hosts Strain construction report
|
|
PCR verification
|
|
Strain purification
|
|
Strain preservation
|
|
Strain Construction (Yeast)
|
Preparation of competent yeast cells
|
10
|
|
Plasmid linearization
|
|
Electro transformation
|
|
Resistance/Nutritional deficiency screening
|
|
Strain purification
|
|
Strain preservation
|
|
Suitable Host Screening
|
Plasmid or Genome DNA extraction
|
15-20
|
Host screening processCOA of engineered strains
|
|
PCR verification, restriction enzyme digestion
|
|
Target gene sequencing
|
|
Screening of high-expression hosts
|
|
Screening of hosts with genetic stability
|
|
Strain preservation
|
Case study
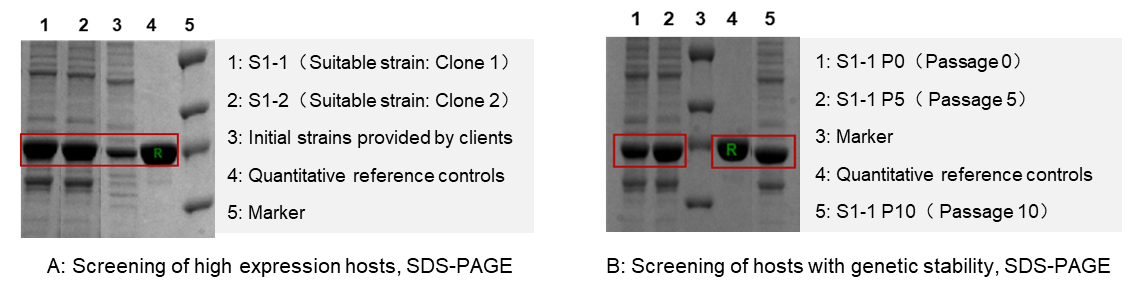
A suitable host improves protein expression level by 2.5 times.
We are commissioned to develop high-producing E. coli strain. And we tried three types of commercial E. coli hosts, based on the characteristics of target protein. It is showed that the strain S1-1 and S1-2 (Fig A: Lane 1,2) improved protein level to 3.5 times than client’s initial strain (Fig A: Lane 3).
Besides, the strain S1-1 performed high genetic stability (Fig B), and it was recognized as candidate strain for primary cell banking (PCB).
Our Service Features
Demonstrated expertise with various microbial hosts, e.g., E. coli DH5α, TOP10, Trans10, BL21; P. pastoris SMD1168H, X-33, GS115, PichiaPink strain1/2/3/4; S. cerevisiae and H. polymorpha.
We are experienced with E. coli periplasmic secretion, soluble and inclusion body expression, as well as yeast intracellular or secretion expression. We add no antibiotics or add the antibiotics under regulatory guidance.
We establish a high throughput screening platform to screen high-producing and stable strains from over one hundred candidate strains.
It is ensured that the process of strain engineering and screening is traceable, under the Good Data and Record Management.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN


