The Significance of Downstream Process (DSP) Development
The whole fermentation broth contains target protein or plasmid, as well as product- and process-related impurities, e.g., aggregates, microbial cell substrates, Host Cell Proteins (HCP), Host Cell DNA (HCD), endotoxin, and antibiotics.
Therefore, it is pivotal to design an efficient DSP, purification process, to remove certain impurities and produce highly purified and high-quality drug substance. Additionally, the optimization of purification process can help to increase product recovery, reduce costs, and improve process scalability and reproducibility.
Keywords: process development, optimization, and validation, process of purification, chromatography, impurities elimination, HCP removal, HCD elimination, endotoxin removal, protein denaturation and refolding, VLP assembly, conjugation
Application: Biopharmaceutical industry, human medicine, animal medicine, vaccine, recombinant large molecule biologics, recombinant biologics, biological reagent
DSP Solutions of Yaohai Bio-Pharma
We have extensive experience isolating target proteins or plasmids from complex matrices by developing and optimizing downstream purification process. There are different types of unit operations suitable for the purification of biologics, including centrifugation, filtration, homogenization, alkaline lysis, ultrafiltration, precipitation, protein denaturation and refolding, chromatography, etc.
Our available purification development services include:
- Development of the entire purification process from cell or supernatant collection to the final active ingredient.
- Optimization of the purification process based on product- and process-related impurities to increase product quality and safety, e.g., HCP, HCD, endotoxin.
- Definition and optimization of key parameters in one or several unit operations, including cell lysis, tangential flow filtration, resin screening, chromatography, protein denaturation and refolding, etc.
- Optimization of process in terms of quality, yield, recovery, cost, and scalability.
- Validation of the downstream purification process.
- Risk-based parameter range assessment and Design of Experiments (DoE) in process modeling.
Service Details
Standardized protein or plasmid purification platforms are based on multiple crude purification steps and less than 4 chromatography steps, including capture, intermediate purification, and polishing.
| Service Details |
Unit Operations |
Parameters |
| Crude Purification |
Centrifugation |
Rotational Speed, Time |
| High-pressure Homogenization |
Total Solid Content, Pressure, Cycles |
| Continuous Alkaline Lysis |
The ratio of Resuspended Cells to Lysis Solution, Lysis Time |
| Tangential Flow Filtration |
Membrane Material and Pore Size, Feed Flow Rate Transmembrane Pressure (TMP), Filtrate volume to membrane area ratio |
| Precipitation |
Precipitant Type and Concentration, Additive, pH, Temperature, Time |
| Denaturation and Refolding |
Inclusion body protein solubilization |
Denaturant Concentration (e.g., Urea, Guanidine HCl, Strong Ionic Detergent), Detergents (e.g., Sodium dodecyl sulfate, SDS), Reducing Agents (e.g., Dithiothreitol, DTT), Chelating Agents (Ethylenediaminetetraacetic acid, EDTA), Temperature, Time |
| Protein refolding |
Refolding Methods (dilution, dialysis or SEC refolding), Protein Concentrations, Buffers, pH, Temperature, Time, Oxidizing and Reducing Agents (e.g., Glutathione, GSH/ Oxidized Glutathione, GSSH, DTT/GSSH, Cysteine/Cystine), Small Molecule Additives (L-arginine, Urea, Guanidine/HCl, and Detergents) |
| Capture, Intermediate Purification, and Polishing |
AC (Affinity Chromatography) |
Several types of chromatography resin/column (e.g., AC, IEX, HIC, SEC, RPC, MMC), Column Length and Diameter, Buffer Composition, Injection Volume, Mobile Phase Composition (Adsorption and Desorption), pH, Flow Rate, Binding, Washing, and Elution Conditions. |
| IEX (Anion or Cation Exchange Chromatography) |
| HIC (Hydrophobic Interaction chromatography) |
| Desalting and/or size exclusion chromatography (SEC) |
| RPC (Reversed Phase Chromatography) |
| MMC, Mixed mode chromatography |
Case study
Case 1
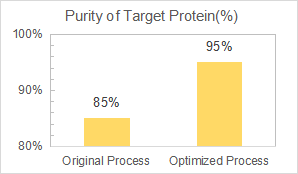
We are commissioned to develop and optimize the downstream purification process to increase the protein purity from 85% to 90% while reducing chromatography steps.
Yaohai delivered a robust and scalable purification process with 3 steps of chromatography for the target protein, a bacterial allergy. And the final purity reached 95%.
Case 2
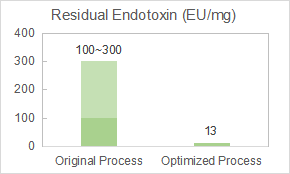
Yaohai was appointed by our client to optimize the endotoxin removal process of the virus-like particle (VLP) vaccine.
Our team performed chromatography parameters optimization and prepared a drug substance with purity of 98% and endotoxin residue of 12.8EU/mg.
Our experiences
- We have been involved in the development and manufacturing of various large molecules, including recombinant subunit vaccines, VLPs, hormones (insulin, GLP-1, growth hormone), cytokines (Interleukin-2/IL-2, IL-15, IL-21), growth factors (EGF, FGF, NGF), nanobodies/single-domain antibodies (sdAbs), enzymes, etc.
Equipment
We use industry-leading AKTA Pure, AKTA Avant, and Preparative HPLC systems for the performance of various chromatography methods including affinity, ion exchange, hydrophobic interaction, reverse phase, and size exclusion chromatography.




 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN






