אנו מייצרים אנטיגנים טרפונמליים תחת השם המסחרי Yaohai. אלו הם חומרים ייחודיים המשמשים לאבחון ומגמה של כמה בעיות בריאות. לפני שימוש באנטיגן עבור בני אדם, הוא חייב לענות על דרישות בטיחות וביצוע באמצעות תהליך ייחודי שנקרא ייצור GMP. זהו תהליך חיוני, ובמאמר זה נדון בייצור GMP ולמה הם הכרחיים עבור אנטיגנים טרפונמליים.
GMP הוא קיצור של Good Manufacturing Practice. זו רשימה של כללים ותקנות חיוניים שאספות כמו Yaohai PhD עוקבות להבטיח שכולם יוכלו להפיק תועלת מהתוצרים שלהם בדרך האפקטיבית והבטוחה ביותר. בעולם האנטיגנים הטרפונמליים, התאמה ל-GMP אומרת שאנחנו מבטיחים שהאנטיגנים שלנו הם טהורים, בטוחים ויעילים. זה אומר שהם לא מעורבים עם חומרים כימיים או זיהומים לא נעימים, ועובדים כפי שאמור להיות.
הנחיות אלה עוזרות לנו לחסוך כסף עבור החברה וגם למנוע איום שיעלה אם האנשים משתמשים באנטיגנים האלה יסבלו. החברה משתמשת ב-GMP כדי שיידעו מה נמצא בתוכם של המוצרים שלהם. התוצאה של זה יכולה להיות לקוחות שמחים יותר שיש להם אמון גדול יותר במוצרים שהם משתמשים בהם. למטופים שצרך את האנטיגנים האלה, GMP הוא חשוב כדי לוודא שהבדיקות הן מדויקות ומאובטחות. מה שאומר שרוב בעלי החיים המזון המקומיים יכולים לבטוח שהבדיקות עובדים טוב, ואם הם זקוקים לטיפול.
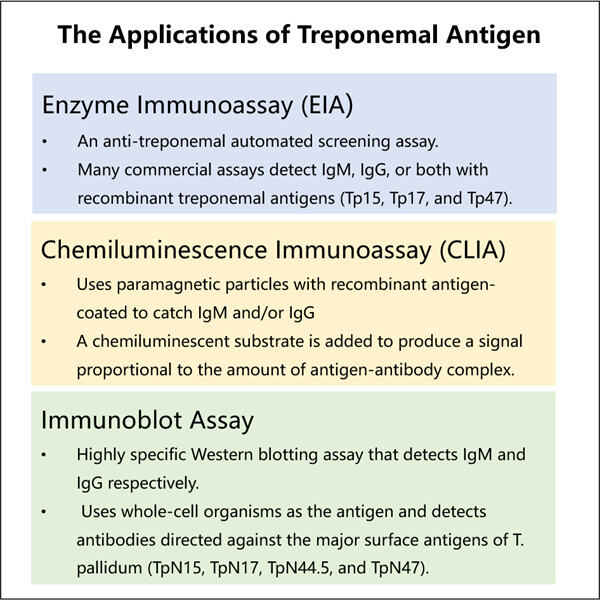
אנטיגנים טרפונמליים דורשים איכות די גבוהה [26]. איכות פירושה שאנטיגנים אלה טהורים ועוסקים כמו שצריך. כאן נכנס לתמונה ביצוע זכרון מוגן (GMP) כדי להציל את היום.ßerdem, כדי לייצר אנטיגנים אלו כל מה שאנו משתמשים בו צריך לעבור בדיקות ובדיקות מתודיות לפי חוקי GMP, מהכנת החומרה עד הכלים שנמצאים בשימוש. דirektives מסוג זה עוזרים לנו לשמור על תוצרתנו נקיה ובאיכות הגבוה ביותר האפשרי. זה גם אומר שאנשים יכולים להיות בטוחים שהאנטיגנים הטרפונמליים שלנו הם בטוחים ויעילים לחלוטין,
אלו אינם היתרונות היחידים של אימוץ GMP כדי להכין אנטיגנים טרפונמליים, אך זה יקצר ויסimplify את תהליכי ההכנה של טרפונמות אחרות. חברות יכולות לעבוד בדרך מסוימת, כאשר יש להם חוקים קבועים והחומרים והכלים הם אותם בכל פעם. זה מאפשר להם להכין את האנטיגנים בצורה מהירה יותר ובאופן כלכלי יותר. זה קריטי עבור אנטיגנים טרפונמליים כי זה יאפשר לבנות מספר גדול של אנשים לעבור בדיקה, וגם בזמן קצר יותר. באופן כללי, ככל שנוכל להכין את האנטיגנים הללו מהר יותר, כך יתכן ליותר אנשים להפיק תועלת מהם.
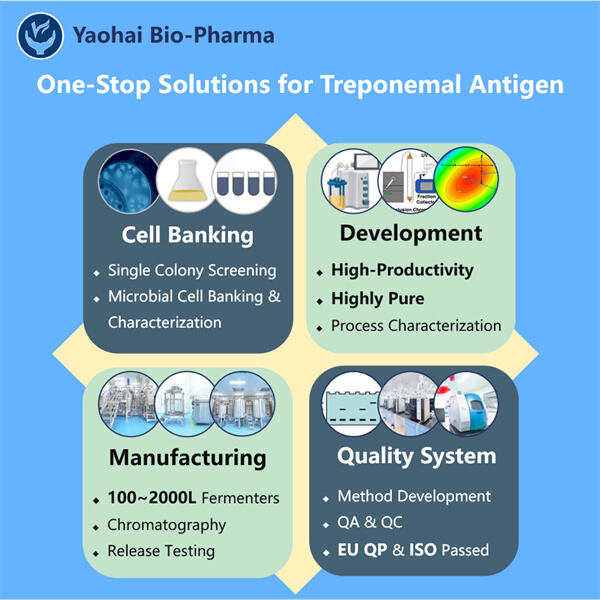
ייצור אנטיגן Trponemal GMP הוא אחת מ-10 החברות הביוטכנולוגיות המובילות העוסקות בפרוקריוטים תרביית. בנו מתקן מודרני עם יכולות חזקות של מחקר ופיתוח (R&D) ותשתית ייצור מודרנית. חמש קווי ייצור לפהמות לפי תקן GMP עבור טיהור ותרביית מיקרואורגניזמים, ושני קווי מלאי אוטומטיים לבקבוקים וקרטידжи ולמחטים מוכנות. סケלות התרבית הזמינות הן 100L, 500L, 1000L ועד 2000L. תקן למלאי בקבוקים משתנה בין 1ml עד 25ml. תקן למלאי בסירוגים מוכנים וקרטידגים הוא בין 1-3ml. המפעל לייצור הוא תואם את תקן cGMP, והוא מבטיח מספק יציב של מוצרים מסחריים ודגימות קליניות. המפעל שלנו מייצר מולקולות גדולות שמופרות ברחבי העולם.
ייצור אנטיגן טרפונמלי GMP הוא אחת מ-10 המובילות בתחום המיקרוביולוגי של CDMO שמשלבת בקרת איכות ופרטים רגולטוריים. הקמנו מערכת איכות חזקה שתואמת את תקני ה-GMP הנוכחיים והתקנות ברחבי העולם. צוות ההדרכה שלנו מכיר היטב את המסגרות הרגולטוריות העולמיות כדי להאיץ את השיחות הביולוגיות. אנו מבטיחים תהליכי ייצור ניתנים לעקיבה ותוצרת באיכות גבוהה שתואמת את הכללים של US FDA, EU EMA, Australia TGA ו-China NMPA. Yaohai BioPharma עברה בהצלחה ביקורת באתר שנערכה על ידי אדם מוסמך מאיחוד האירופי (QP) כדי לבחון את מערכת ה-GMP שלנו ואת מתקן הייצור שלנו. בנוסף, עברנו בהצלחה את הביקורות הראשונות של מערכות ניהול איכות ISO9001, ניהול סביבתי ISO14001 וניהול בריאות ובטיחות תעסוקה ISO45001.
Yaohai Bio-Pharma היא מובילה בתחום CDMO של ביולוגיים מיקרוביים. התמקדותנו העיקרית היא ביצור חיסונים ותרפיות מיקרוביות לניהול בעלי חיים, בני אדם ו-Manufacturing תחת GMP של אנטיגן טרפוןמלי. אנו מצוידים בפלטפורמות RD מתקדמות ובתכנולוגיות ייצור שמכסות את כל התהליך, החל מהפיתוח של אצגוני מיקרובים ובנקאות תאיות, דרך פיתוח שיטות והעלאה על הרגל, ועד לייצור קליני ומסחרי כדי להבטיח את ספקת הפתרונות החדשים בהצלחה. עם הזמן, צברנו ידע רב על עיבוד ביולוגי מבוסס מיקרובים. יותר מ-200 פרויקטים הושלמו בהצלחה ואנחנו מסייעים לתורנים שלנו לעבור את הרגולציות, כמו אלו של US FDA ו-EU EMA. אנו גם מסייעים להם לנווט את TGA של אוסטרליה ואת NMPA של סין. אנו יכולים להגיב במהירות לצרכים השווקיים ולהציע שירותים מותאמים של CDMO בזכות החוויה והידע שלנו.
Yaohai Bio-Pharma מייצרת אנטיגן טרפונמלי GMP מביולוגיים מיקרוביאליים. אנו מציעים פתרונות מותאמים של מחקר ופיתוח וכן ייצור, תוך שמינון הסיכונים. היינו מעורבים במספר מודליזים כמו חיסונים תת-יחידה רקורסיביים, הורמונים פפטידיים, ציטוקינים גורמי צמיחה, חסמים חד-דומייניים, אנזימים, DNA פלסמידי, mRNA ועוד. אנו מומחים במספר מארחי מיקרובים, כמו שמרים חיצוניים ובפנים תאיים (הפקה עד 15 גרם לליטר), סקירת בקטריה פריפלזמית וכן גופי כלולות דיסולביליים ותאתיים (הפקה עד 10 גרם/ליטר). בנוסף, פיתחנו את פלטפורמת התסיסה המיקרוביאלית BSL-2 לפיתוח חיסונים חיידקיים. יש לנו תיעוד של לשיפור תהליכי ייצור, מה שמציב את התוצאות ומחוסך עלויות. עם צוות טכנולוגי יעיל במיוחד, אנו מבטיחים מסירה מהירה ובטוחה של פרוייקטים והופכים את המוצרים שלכם לשווקים מהר יותר.