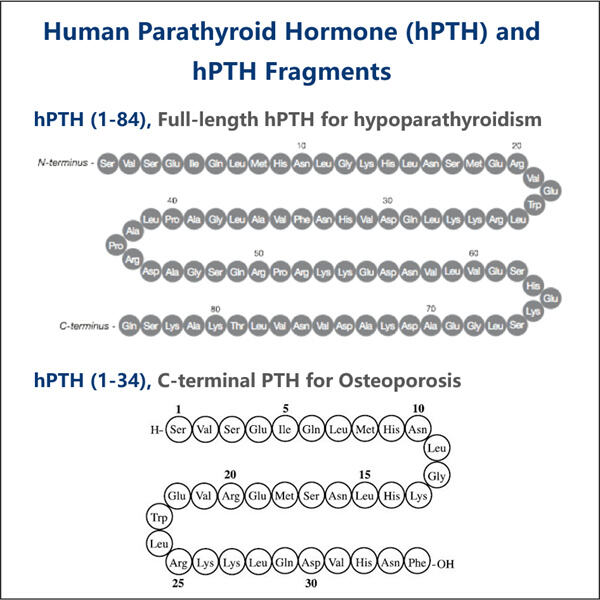
組み換えとは、異なる成分を組み合わせることを意味します。PTH-副甲状腺ホルモン1:カルシウム濃度を調節するために体内で働くホルモン。次に、「製造」という言葉が何であるかは誰もが知っています。機械と技術から何かを作ることです。これらすべての言葉を組み合わせると、Yaohaiが生まれます。 組換え豚インスリンのバイオ製造 そして、その部品を巧みに組み合わせることで、機械を使用して副甲状腺ホルモンを製造することと定義できます。
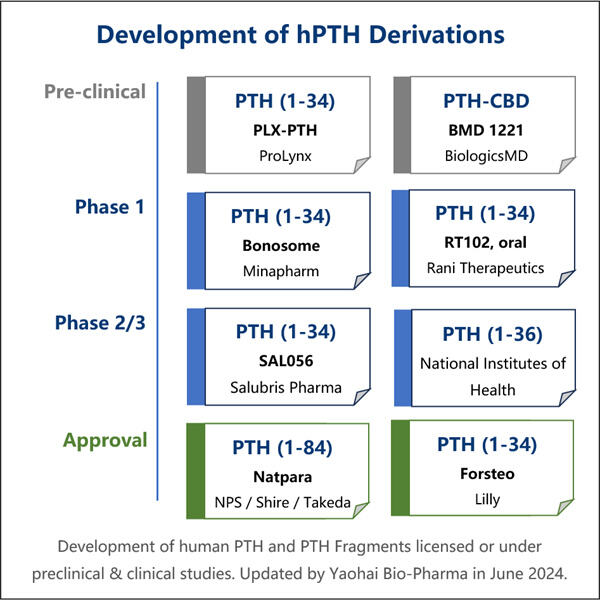
PTH は、科学者が長年かけて開発した、また新しい組み換え型です。これは、バクテリアと呼ばれる小さな生物によって使用され、副甲状腺ホルモンの生成に特定のタンパク質を使用します。この市場リーダーの 1 つが Yaohai です。同社は、組み換え型 PTH の独自の革新的なプロセスを開発しました。
バクテリアを調理するために、彼らはまず DNA を準備します (この魔法のレシピ本は、いつどこでバクテリアにスイッチを入れるかを教えてくれます)。次に、この DNA をバクテリアに注入します。そして、研究室でこれらのバクテリアを培養します。Yaohai GMP 組み換えペプチド製造 PTH ホルモンを放出し、それが人間と同じように繁殖を促し、より多くの PTH を生成します。そのため、錬金術はこのホルモンを大量に摂取する効果的な方法です。
仰向け PTH 生産 さらに、このリソースは、消費者にとって PTH 製品の品質を備えたより手頃な方法を生み出すのにも役立ちます。 PTH は、動物と人間の PTH に基づいているため高価な従来の方法で生産されます。 これは、特に大量のホルモンを合成する必要がある場合、時間がかかり、リソースを大量に消費するプロセスになる可能性があります。
対照的に、組み換え PTH の製造は難しく、費用もかかります。ただし、このホルモンはバクテリアを使って作られます。組み換え PTH を生成する新しいプロセスは、より速く、よりシンプルなプロセスです。これにより、それぞれの医師は、取り扱い、調達、投与に費用がかからないため、PTH 治療を低価格で提供できます。つまり、より多くの人々が、高額な料金をあまり気にせずに、受けるべき治療を受けられるようになるのです。
これは医学分野における画期的な進歩と言えるでしょう。組み換え PTH 製造法が誕生したのです。この方法により、治療に不可欠な PTH ホルモンをはるかに簡単に、そして安価に製造できるようになりました。患者は、費用をあまり気にすることなく治療を受けることができます。ニュースによると、このような治療を必要とする多くの人々が、この技術に大いに安堵したそうです。

組み換えPTHは予測可能で、これが次の大きな利点につながります。従来のPTH(例えば、複数のソースから抽出され、その結果変化することが多い)とは異なり、従来のPTHとは結果が異なる可能性があります。Yaohai 組み換えパーキンタンパク質の製造 管理された環境で製造されているため、毎回品質結果が保証されます。定期的な治療を必要とする患者は、この一貫性に真の価値を感じることができます。
Yaohai Bio-Pharma は、組換え PTH 製造 CDMO のリーディングカンパニーです。当社は、ペット、人間、動物の健康を治療するための微生物ワクチンと治療薬の製造に主眼を置いています。当社は、微生物株のエンジニアリングから細胞バンキング処理、方法設計、臨床および商業製造まで、製造プロセス全体をカバーする最先端の RD および製造技術プラットフォームを所有しており、最先端のソリューションを確実に提供できるよう努めています。当社は、バイオ処理微生物分野で膨大な知識を蓄積してきました。200 件を超えるプロジェクトを成功裏に完了しており、お客様が米国 FDA や EU EMA などの規制に準拠できるよう支援しています。また、オーストラリア TGA や中国 NMPA への対応も支援しています。当社は、経験と専門知識を活かして、市場の要求に迅速に対応し、カスタマイズされた CDMO サービスを提供することができます。
組み換えPTH製造は、微生物由来の生物製剤の製造経験があります。当社は、リスクを最小限に抑えながら、カスタマイズされたRDおよび製造ソリューションを提供します。当社は、ワクチンの組み換え細胞サブユニット(ペプチドを含む)、成長因子、ホルモン、サイトカインなど、さまざまな技術を試してきました。当社は、酵母の細胞外および細胞内分泌物(最大15g/Lの収量)や細菌の細胞内可溶性および封入体(最大10g/Lの収量)など、複数の微生物を専門としています。また、細菌ワクチンを開発するためのBSL-2発酵プラットフォームも備えています。当社は、プロセスの改善、製品収量の増加、および製造コストの削減の専門家です。効果的な技術チームにより、タイムリーで質の高いプロジェクト配信を保証し、製品をより早く市場に投入します。
Yaohai Bio-Pharma は、生物製剤のトップ 10 製造業者であり、微生物発酵のスペシャリストです。当社は、最新の設備と強力な RD 製造能力を備えた高度な施設を設立しました。当社には、微生物発酵と精製の GMP 要件に準拠した 100 つの薬物物質製造ラインと、カートリッジ、バイアル、プレフィルド シリンジ用に自動化された 500 つの充填仕上げラインがあります。利用可能な発酵スケールは、1000 L から 2000 L、1 L、25 L までです。バイアル用の組み換え PTH 製造は 1 ml から 3 ml ですが、プレフィルド シリンジとカートリッジ充填仕様は XNUMX-XNUMX ml をカバーしています。当社の生産ワークショップは cGMP に準拠しており、臨床サンプルと商用アイテムの安定した供給を保証します。当社の工場は、世界中に出荷される大きな分子を生産しています。
Yaohai BioPharma は、品質管理と規制の問題を組み込んだ上位 10 位の微生物 CDMO です。当社は、世界中の現在の GMP 基準と規制に準拠した強固な品質管理システムを開発しました。当社の規制チームは、世界中の規制の枠組みを深く理解しています。これにより、生物学的製品の発売を加速できます。当社は、追跡可能な製造プロセスと高品質の製品を確保し、米国 FDA と EU EMA のガイドラインに準拠しています。組み換え PTH 製造と中国 NMPA も満たしています。Yaohai BioPharma は、当社の GMP システムと製造施設を審査するために、欧州連合の認定資格者 (QP) が実施した現地監査に合格しました。当社はまた、ISO9001 品質管理システムと ISO14001 環境管理システムの最初の認証監査も受けています。