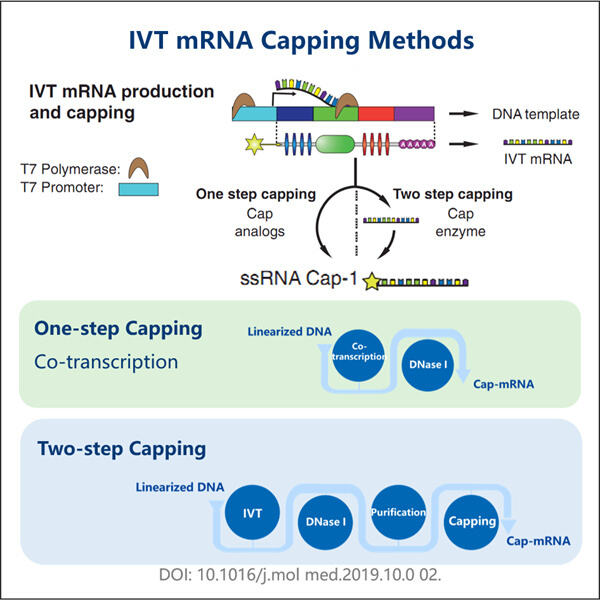
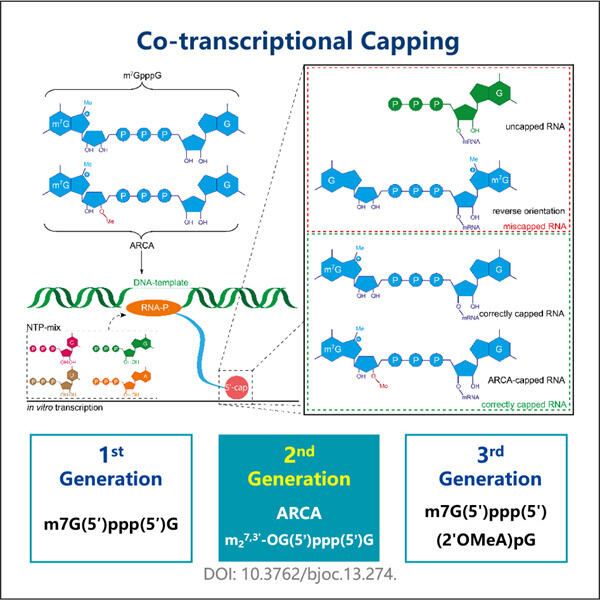
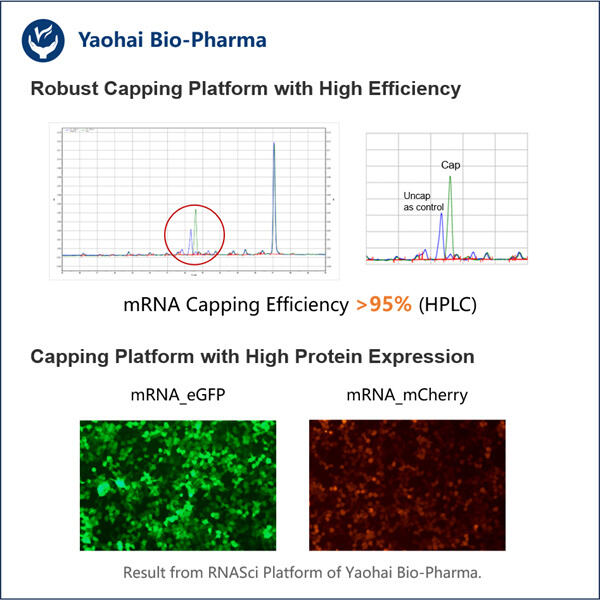
Tecnica Migliorata di Capping Co-trascrizionale
La cappatura in questo caso avviene molto spesso dopo la conversione da DNA a mRNA, nonché il Fornitore GMP GLP-1 Sema innovato da Yaohai. E viene chiamata Trascrizione. Ma è un po' un processo lungo e dura per sempre. Il nostro nuovo approccio ci consente di bloccare l'mRNA dall'essere cappato, proprio mentre viene creato. Questo fenomeno è chiamato cappatura co-trascrizionale, e permette all'intero processo di procedere più velocemente. Entrambe le azioni porteranno a uno sviluppo più rapido e ottimizzato quando vengono eseguite insieme.
Eseguiamo questo nuovo approccio con un piccolo aiuto da un aiutante molto speciale (un mimico del trifosfato). Questo chaperon è coinvolto nel cappatura durante il processo di produzione di mRNA. Questo aiuterà il tuo processo di cappatura. La vera bellezza di questo sistema, però, è che altri chimici possono sintetizzare facilmente questo mimico del trifosfato senza spendere troppo. Un'altra grande soluzione per i laboratori di ricerca che hanno bisogno di produrre grandi quantità di mRNA.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN