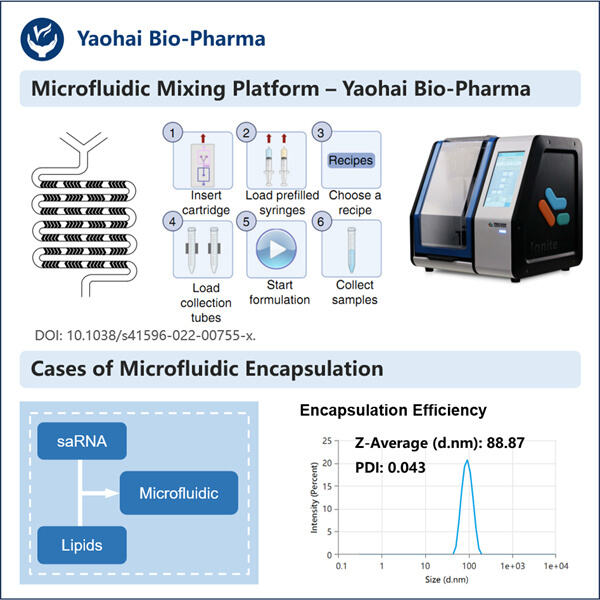
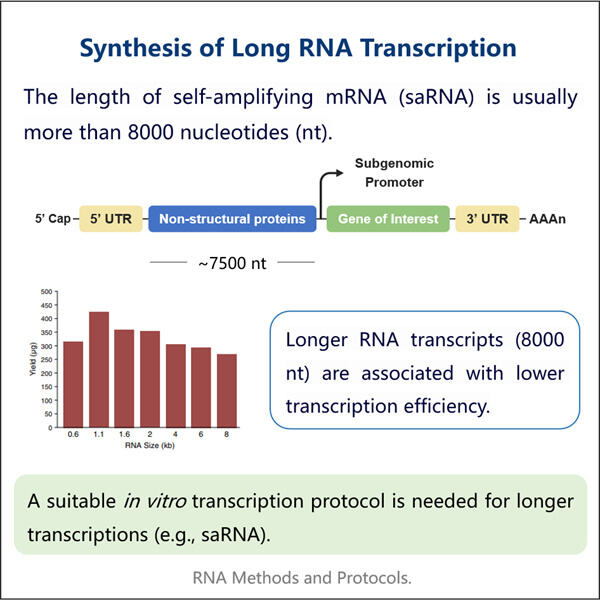
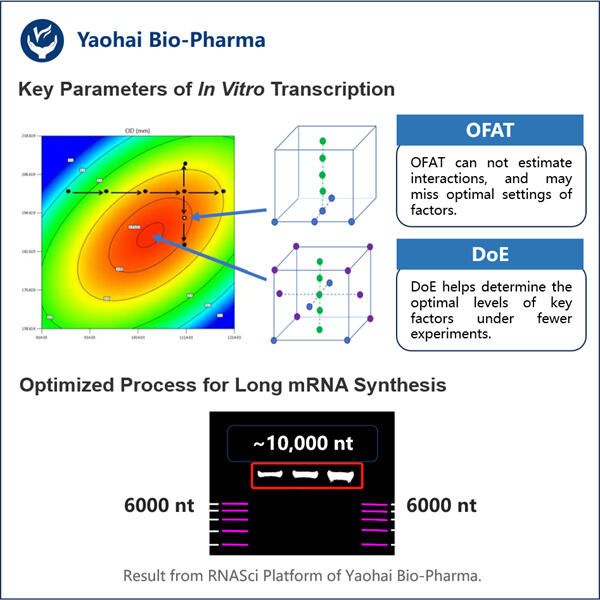
Heute ist Yaohai so aufregend, sein In-vitro-Transkriptionsprotokoll für lange RNA zu teilen. Es ist eine effektive Strategie, die Wissenschaftler häufig verwenden, um RNA im Labor herzustellen. Auf diese Weise können Forscher eine signifikant große Menge an langer RNA herstellen – etwas, das für die Forschung in verschiedenen biologischen Bereichen von entscheidender Bedeutung ist. Hier bieten wir eine Schritt-für-Schritt-Anleitung für die Herstellung langer RNAs durch In-vitro-Transkription. Wir werden auch darüber sprechen, wie wir den Prozess verbessern können, und versuchen, einige der Probleme zu lösen, mit denen Sie bei der Synthese langer RNA konfrontiert sind.
Für die In-vitro-Transkription werden mehrere wesentliche Komponenten benötigt, insbesondere lange RNA. Sie müssen ein Enzym namens RNA-Polymerase, Schreibmaterial in Form von Nukleotidtriphosphaten (NTPs), einen Puffer zur Unterstützung der Reaktion und eine DNA-Vorlage bereitstellen. Eine detaillierte Anleitung, wie Sie Schritt für Schritt vorgehen, finden Sie hier:

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NEIN
NEIN
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN