Hjælper Dig med at Forstå CMO, CDMO, og CMO Hurtigt
Inden for farmaceutisk og bioteknologisk industri er valget af den rigtige partner til udvikling og produktion af lægemidler afgørende. Selv om Contract Research Organization (CRO) og Contract Manufacturing Organizations (CMO) tilbyder dygtige tjenester, giver Contract Development and Manufacturing Organization (CDMO) en mere integreret tilgang, der kan forenkle hele processen.
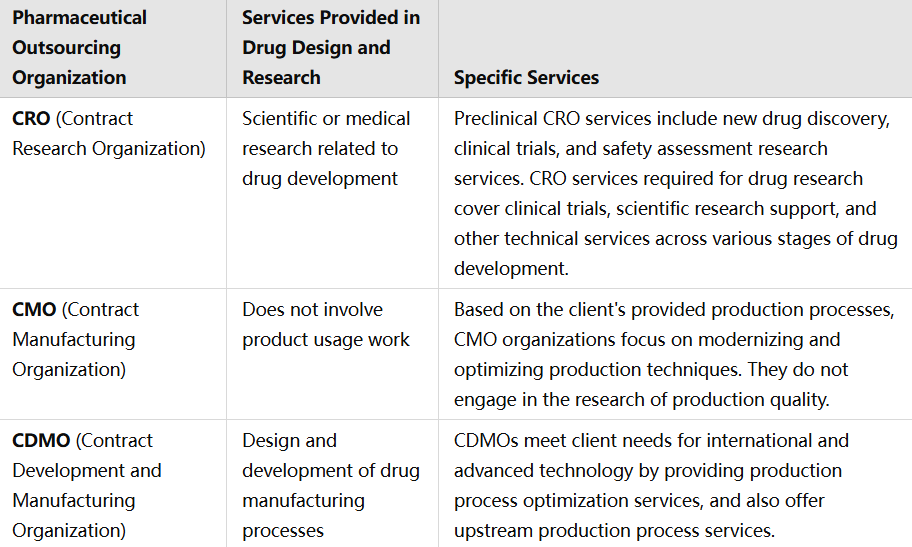
Forståelse af CRO, CMO og CDMO:
Contract Research Organization (CROs): CRO fokuserer på forskningsfasen og tilbyder tjenester såsom ledelse af kliniske prøver, præklinisk forskning, dataadministration og reguleringssager. De koncentrerer sig hovedsageligt om de tidlige faser af lægemiddeludviklingen.
Contract Manufacturing Organization (CMO): CMO fokuserer på produktionen og håndterer storstilforsyningen når et lægemiddel er blevet udviklet. Deres tjenester omfatter kommersiel skala-produktion, pakning og kontroltest af kvalitet.
Contract Development and Manufacturing Organization (CDMO): CDMO tilbyder løsninger fra begyndelsen til slutningen, dvs. både udvikling og produktion. De tilbyder services fra formuleringudvikling og procesoptimering til produktion af klinisk prøve-materialer og kommersiel produktion. Denne integrerede tilgang mindsker behovet for flere partnere og forenkler udviklingsprocessen.
Serviceforskelle i CRO, CMO, CDMO:
Forholdet mellem CDMO og salgsprodukter
CDMO-services er afgørende for leveringen af det endelige produkt. Lægemiddeludvikling er en højrisiko, højinvestering, langtids-process. For at reducere omkostninger og forbedre effektiviteten outsourcerer farmaceutiske virksomheder lægemiddelprocesudvikling og produktion til CDMOs. Services inkluderer typisk procesdesign, opskalering, strukturbekræftelse, stabilitetsstudier, snavsanalyse og tilpasset produktion. Efter færdiggørelse leveres mellemprodukter eller APIs til kunder.
- CDMO-services sikrer den succesfulde udvikling af kommersialiserbare mellemprodukter eller APIs.
- CDMO-tjenester er afgørende for at opfylde de specifikke krav til tilpassede farmaceutiske produkter.
- Endkunder deltar i kvalitetsauditorier for at sikre sikkerhed, effektivitet og kvalitet.
- CDMO holder sig til branches standarder, hvilket sikrer konsekvent service og levering.
Med årtiers dedikeret indsats har Yaohai Bio-Pharma etableret en førende en-stop CRO/CDMO/MAH-serviceplatform i branchen. Indtil nu har firmaet succesfuldt afleveret over 200 projekter, heraf 3 kliniske fase III-forsøg, 4 fase II-forsøg, flere IND og fase I-kliniske forsøg.
Af disse er 7 projekter dobbelt indsendt i både USA og Kina, og 2 er registreret i Australien. Projekterne dækker en række hovedstrømsbiologika og terapeutiske indikationer, hvilket opfylder reguleringssubmissionskravene i flere globale regioner.
Vi søger også aktivt institutionelle eller individuelle globale partnere. Vi tilbyder den mest konkurrencedygtige kompensation i branchen. Hvis du har nogen spørgsmål, tøv ikke med at kontakte os på [email protected]
Nyheder
-
Yaohai Bio-Pharma Bestod EU QP Audit og Opnår ISO Triple Certificering
2024-05-08
-
BiotechGate, Online
2024-05-13
-
2024 VERDENSDAG FOR VACCINER Washington
2024-04-01
-
CPHI Nordamerika 2024
2024-05-07
-
BIO International Convention 2024
2024-06-03
-
FCE COSMETIQUE
2024-06-04
-
CPHI Milan 2024
2024-10-08

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN

