يُشير اختصار CMC إلى الكيمياء، والتصنيع، والتحكم. يمكن أن يكون هذا المكون مكونًا مهمًا لمعالجة تطبيق هادئ لدواء جديد. يخبرك كيفية تصنيع الدواء وكيف تم اختباره للتأكد من أنه خيار علاجي آمن وذو جودة عالية للأشخاص الذين سيتناولون هذا الدواء. فهم ما يعنيه شرح Yaohai CMC. اختبار كفاءة تغطية MRNA للحمضات النوويّة الحلزونيّة (Plasmid DNA) قد يساعد في تقديم رؤية جديدة حول كيفية ضمان الشركات لجودة منتجاتها.
هناك قواعد دقيقة لتقديم دواء، تحتاج الشركات إلى إثبات أن الدواء تم تصنيعه بشكل صحيح وأنه مناسب للاستخدام. تحتوي إدارة الأغذية والأدوية FDA، التي هي اختصار لـ Administration and Food Drug، على إرشادات حول كيفية إعداد وتقديم طلبات الموافقة على الأدوية. تضمن هذه القواعد سلامة الجمهور وتؤكد أن الأدوية الآمنة فقط هي المتاحة.
وهذا لأن أحد أهم المبادئ الأساسية لموافقة الأدوية هو إثبات أن المنتج نقي ولديه فعالية موثوقة. لذلك، يجب تنظيم عملية إنتاج الأدوية بشكل صارم، وهذا هو السبب في أن بعض شركات الأدوية ذات التشابه في النسخة s593 عملت معًا لمدة تزيد عن عقد من الزمن لتقديم الأدوية. وهذا يتضمن مرور الشركات عبر عملية خطوة بخطوة لضمان أن المنتج النهائي يتم الاحتفاظ به وفقًا للمعايير عالية الجودة. وهذا أمر أساسي لأن حياة الناس تعتمد على الأدوية التي يستهلكونها.
يجب أن تقدم الشركات التي تسجل طلبًا لدواء بيانات تفصيلية حول ما يدخل في الدواء وكيفية صنعه. وهذا يتضمن التركيب الكيميائي، عملية التصنيع، والمعايير الأمنية المدمجة فيه. يتم دعم هذه البيانات أيضًا بالأدلة العلمية - مما يثبت أن الدواء آمن وفعال. كلما كانت إدارة الأغذية والأدوية (FDA) قادرة على فهمها بشكل أفضل، بروتوكول تغطية الترجمة المشتركة للـ MRNA يمكنهم مقارنة بياناته بما تم اختباره في دراسات أخرى (تحليل متعدد الدراسات) في أماكن مختلفة.
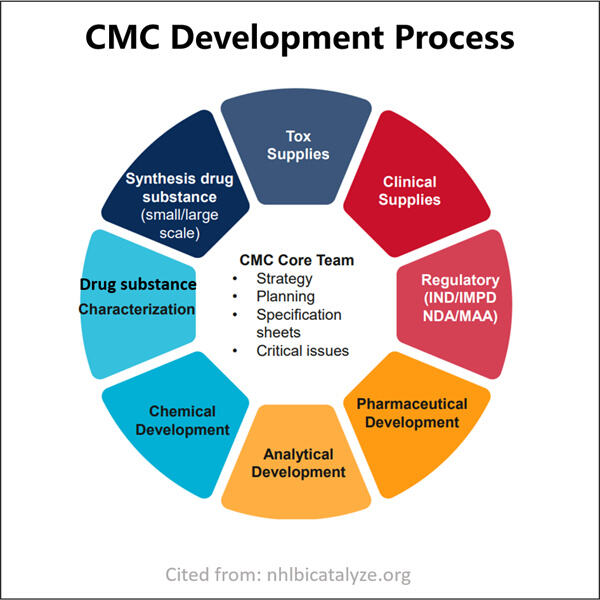
لذلك، يجب أن يجتاز كل دواء جديد العديد من الاختبارات التي تقوم بها الشركات للتأكد من أنه يعمل وأنه آمن لجسم الإنسان. وبالتالي، سيتم اختبار هذا الدواء على الحيوانات لتحليل كيفية تصرفه داخل الحيوانات الحية. بعد ذلك يمكن إجراء التجارب على البشر لمعرفة ما إذا كان الدواء يعمل، سواء على المدى القصير أو الطويل. تساعد مثل هذه الاختبارات الباحثين في تحديد كيفية عمل الدواء الجديد Yaohai. بروتوكول التغطية الإنزيمية للـ MRNA يؤثر على الجسم وما إذا كان هناك آثار جانبية أم لا.

في حالة رغبتك في زيادة فرصك في الحصول على الموافقة، قم بتقديم المعلومات الأساسية دفعة واحدة. الطلب غير المنظم أو المليء بالأخطاء سيؤدي إلى مراجعته مرة أخرى وربما رفضه. عادةً ما يكون هذا السبب ياوهاي اختبار سلامة MRNA الشركات حذرة للغاية وتتحقق مرتين مما يؤدي إلى بطء عملية الشراء لشركائهم.

النظام الكهربائي تطوير عملية البلازميد المرسال (mRNA) جزء من طلب الدواء هو أمر أساسي لأمان وفعالية الحلول الحديثة. من خلال الالتزام بالإرشادات الصارمة وكشف جميع البيانات، يمكن لمصنعي الأدوية إثبات أن أدويتهم تستطيع تحقيق أعلى المعايير. هذه العملية ضرورية لحماية صحة الأفراد.
يتمتع Yaohai Bio-Pharma بخبرة واسعة في المنتجات البيولوجية المستخلصة من مصادر دقيقة. نقدم حلول RD المخصصة والتصنيع، مع تقليل المخاطر المحتملة. عملنا على أنماط مختلفة، بما في ذلك اللقاحات الفرعية المركبة، والهرمونات الببتيدية، والسيتوكينات عوامل النمو، والضدود ذات المجال الواحد الإنزيمات، والحمض النووي البلازميدي الحمض النووي الريبوزي المتنوع، وغيره. تخصصنا يشمل العديد من الكائنات الدقيقة، بما في ذلك قسم CMC في طلب الدواء السكريات داخل الخلايا وأيضًا خارجها (الإنتاج يصل إلى 15 جم/لتر) والبكتيريا القابلة للذوبان داخل الخلية وجسم الشمول (الإنتاج يصل إلى 10 جم/لتر). كما أنشأنا منصة تخمير BSL-2 لإنشاء لقاحات قائمة على البكتيريا. نحن خبراء في تحسين العمليات، وزيادة إنتاج المنتجات، وتقليل تكاليف الإنتاج. لدينا فريق تقني ذو كفاءة عالية يضمن تسليم المشاريع في الوقت المحدد وبجودة عالية. هذا يمكّننا من تقديم منتجاتك الفريدة إلى السوق بشكل أسرع.
ياوهاي بايو-فارما هي شركة رائدة في مجال خدمات التطوير والتصنيع المتعاقدة (CDMO) للأدوية الحيوية الدقيقة. كان تركيزنا الأساسي على إنتاج قسم CMC في طلب تسجيل الدواء والعلاجات لرعاية الحيوانات الأليفة، الصحة البشرية والبيطرية. نحن نمتلك منصات بحث وتطوير متطورة وتقنيات تصنيع تغطي العملية الإنتاجية بأكملها، بدءًا من تطوير سلالات دقيقة، بنوك الخلايا، تطوير العمليات والطرق، وحتى التصنيع السريري والتجاري مما يضمن تقديم حلول مبتكرة بنجاح. مع مرور الوقت، اكتسبنا خبرة واسعة في معالجة البيولوجيات القائمة على الكائنات الدقيقة. لقد أنجزنا بنجاح أكثر من 200 مشروع عالمي، ونساعد عملاءنا في التعامل مع القوانين واللوائح الخاصة بالهيئة الأمريكية للرقابة على الأغذية والأدوية (FDA)، وكالة الأدوية الأوروبية (EMA)، وهيئة تنظيم الأدوية الأسترالية (TGA)، والهيئة الوطنية لإدارة المنتجات الطبية في الصين (NMPA). بسبب خبرتنا ومهارتنا، يمكننا الاستجابة بسرعة لمتطلبات السوق وتقديم خدمات CDMO مخصصة.
ياوهاي بيوفارما، قسم CMC في طلب الدواء ميكروبيال CDMO، يدمج شؤون التنظيم والجودة. لدينا نظام جودة متوافق مع معايير GMP الحالية، وكذلك اللوائح في جميع أنحاء العالم. فريقنا التنظيمي على دراية بالإطارات التنظيمية العالمية لتسريع إطلاق المنتجات البيولوجية. نحن نتأكد من أن عمليات الإنتاج قابلة للتتبع، وأن المنتجات ذات جودة عالية وتتوافق مع قواعد FDA الأمريكية وEMA الأوروبية. كما يتم الالتزام بمتطلبات TGA الأسترالية وNMPA الصينية أيضًا. لقد نجحت ياوهاي بيوفارما في اجتياز التدقيق الميداني للشخص المؤهل (QP) في الاتحاد الأوروبي لضمان نظام الجودة الخاص بنا وفقًا لنظام GMP وموقع الإنتاج. كما اجتزنا أيضًا التدقيقات الأولية لنظام إدارة الجودة ISO9001 ونظام إدارة البيئة ISO14001.
ياوهاي بايو-فارما، واحدة من أكبر 10 أقسام CMC في طلبات الأدوية للمنتجات البيولوجية، هي متخصصة في التخمير المجهرى. لقد أنشأنا منشأة حديثة تمتلك قدرات بحث وتطوير قوية وبنية تحتية متقدمة. هناك خمس خطوط إنتاج للأدوية وفق معايير GMP لتنقية وتخمير الخلايا الدقيقة، بالإضافة إلى خطين لإكمال التعبئة للزجاجات والكروتيدجات والإبر المسبقة التعبئة. تتراوح أحجام التخمير المتاحة بين 100 لتر و2000 لتر. مواصفات التعبئة للزجاجات تتراوح بين 1 مل و25 مل، بينما تكون متطلبات التعبئة للإبر أو الكروتيدجات المسبقة التعبئة بين 1-3 مل. ورشة الإنتاج معتمدة حسب cGMP وتقدم عينات تجارية وسريرية. الجزيئات الكبيرة التي يتم تصنيعها في منشأتنا متاحة للتوزيع عالميًا.