As a hormone with 33 amino acids, GLP-2 is a peptide-encoded carboxyterminal to the sequence of GLP-1 in the precursor gene. Since GLP-2 was originally reported to stimulate intestinal growth, there has been huge interest in the potential therapeutic uses for treating diseases such as short bowel syndrome (SBS), in which the loss of a large section of bowel due to intestinal resection occurs.
GLP-2 Analogs for Therapeutic Use
However, the short half-life of native GLP-2 requires constant infusion, thus limiting their use as therapeutic agents. To address this problem, novel GLP-2 agents have been developed, one of which is already commercially available (teduglutide), and others (apraglutide and glepaglutide) are in the late stage of clinical development.
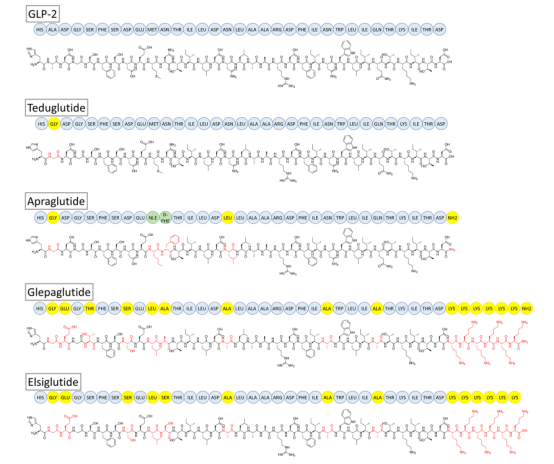
Fig. Sequence of GLP-2 and GLP-2 analogs (Teduglutide, Apraglutide, Glepaglutide, Elsiglutide).
Teduglutide
The active ingredient of teduglutide is [gly2]-hGLP-2, a recombinant GLP-2R agonist based on the 33-amino acid natural hormone. Teduglutide was developed by Shire (now acquired by Takeda) and approved to treat Short Bowel Syndrome (SBS) under the brand names Gattex (USA, FDA) and Revestive (Europe, EMA). The peptide is designed by a single amino acid mutation at the N-terminus (replacing glycine in position 2 with alanine), which makes it less susceptible to degradation by DPP-4 and extends the half-life to about 2 hours.
Glepaglutide
Glepaglutide (ZP1848), with the sequence [Gly2, Glu3, Thr5, Ser8, Leu10, Ala11,16,24,28] hGLP-2(1-33)-(Lys)6-NH2, is a highly potent GLP-2 selective 39 amino acid peptide. Zealand Pharma developed gepaglutide by various peptide design strategies. These strategies include i) six lysine residues are added at the C-terminus to alter the charge state that affect solubility and physicochemical properties; ii) four residues with alanine are substituted in the region connecting the seven transmembrane binding sites to the extracellular structural domain binding residues; iii) N-terminal region is altered and optimized with five changes to improve pharmacokinetic and potency properties.
Apraglutide
Apraglutide (with the code name FE 203799) was initially found by Ferring Pharmaceuticals. The amino acid of native GLP-2 peptide is substituted or modification at position 2, 10, 11, 16, and C terminal to produce apraglutide with the sequence ([Gly2,Nle10, D-Phe11,Leu16]hGLP-2-(1-33)-NH2). In detail, GLP-2 positions 2 and 10 are substituted with glycine and norleucine; and position 11 is hydrophobically substituted with D-phenylalanine; position 16 with leucine and finally C-terminal amidation modification.
Elsiglutide
The sequence of a GLP-2 peptide analogue, Elsiglutide (ZP1846), is [Gly2, Glu3, Ser8, Leu10, Ser11, Ala16, Ala24, Ala28] hGLP-2(1-33)-(Lys)6. It is associated with another GLP-2 compound being developed by Zeeland Pharma. Elsiglutide is developed for the treatment of chemotherapy-induced diarrhea.
Yaohai Bio-Pharma Offers One-Stop CDMO Solution for GLP-2
GLP-2 Analogs Pipelines
|
Generic name
|
Brand Name/Altermative name
|
Expression System
|
Indications
|
Manufacturer
|
Latest stage
|
|
Teduglutide
|
Gly(2)-GLP-2, SHP633, ALX-0600, TAK-63, Gattex, Revestive, レベスティブ
|
E. coli
|
Short bowel syndrome (SBS), malabsorption syndrom
|
Shire, Takeda
|
Approved
|
|
Glepaglutide
|
ZP-1848, long-acting, 39AA
|
Pending Update
|
inflammatory bowel disease, short bowel syndrome, kidney disease
|
Zealand Pharma
|
Submit for approval, Phase III
|
|
HM-15912
|
Fc-coupling protein, chemical conjugation
|
Pending Update
|
Short bowel syndrome (SBS)
|
Hanmi Pharmaceutical
|
Phase II
|
|
SHP-681
|
Fc fusion protein, GLP-2 Analog-Fc Fusion, TAK-681, SHP 681
|
Pending Update
|
Short bowel syndrome (SBS)
|
Shire, Takeda
|
Phase I
|
|
Teduglutide Recombinant
|
Ted, PJ009
|
Pending Update
|
Short bowel syndrome (SBS)
|
Chongqing Paijin Biotechnology
|
Phase I
|
|
GX-G8
|
Long acting GLP-2, Genexine
|
Pending Update
|
Short bowel syndrome (SBS)
|
Genexine, Inc.
|
Phase I
|
|
Apraglutide
|
FE-203799, FE 203799
|
synthetic peptides
|
Short bowel syndrome (SBS)
|
VectivBio, Asahi Kasei
|
Phase III
|
|
Dapiglutide
|
ZP-7570
|
synthetic peptides
|
Obese, Short bowel syndrome (SBS)
|
Zealand Pharma A/S
|
Phase II
|
|
Elsiglutide
|
ZP-1846
|
synthetic peptides
|
diarrhea
|
Zealand Pharma A/S
|
Phase II
|
|
GLP-2
|
Pending Update
|
synthetic peptides
|
Short bowel syndrome (SBS), Intestinal failure
|
Alberta Health Services
|
Phase II
|
|
Long-acting GLP-2
|
Pending Update
|
Pending Update
|
Short bowel syndrome (SBS)
|
OPKO Biologics (formerly Prolor Biotech),
|
Pre-clinical
|
|
Oral GLP-2
|
Pending Update
|
Pending Update
|
Gastrointestinal diseases
|
Applied Molecular Transport, Inc.
|
Pre-clinical
|
|
NM-003
|
GLP-2-XTEN, chemical conjugation, long-acting GLP-2 agonist, NB-1002
|
E. coli
|
Short bowel syndrome (SBS)
|
9 Meters Biopharma, Naia Ltd.
|
Pre-clinical
|
|
PE-0503
|
GLP-2-ELP
|
Pending Update
|
Short bowel syndrome (SBS)
|
PhaseBio Pharmaceuticals
|
Pre-clinical
|
|
GLP-2 Analogs
|
Oral GLP2 analogs
|
Pending Update
|
Gastrointestinal diseases
|
Entera Bio
|
Pre-clinical
|
Reference:
[1] Suzuki R, Brown GA, Christopher JA, Scully CCG, Congreve M. J Med Chem. 2020 Feb 13;63(3):905-927. doi: 10.1021/acs.jmedchem.9b00835.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN


