血友病は、私たちの血液が凝固する仕組みを変える希少な病気です。これは、血友病を持つ人々が関節痛や体内深部の出血などの深刻な困難に直面する可能性があることを意味します。彼らは過剰な出血を防ぐために「凝固因子」と呼ばれる特別な治療を受ける必要があります。これらの治療は非常に効果的ですが、非常に高価でもあります。また、感染症やその他の問題を引き起こす可能性もあります。そのため、専門家たちは血友病の人々を助けるための新しい戦略を探し続けています。その新しい選択肢には、遺伝子療法や「VHHs」と呼ばれる特定のタンパク質があり、これらは「ナノボディ」とも呼ばれています。これらのタンパク質は、私たちの血液の凝固能力を向上させるのに役立ちます。優秀な科学者たちで満ちた国であるヤオハイでは、一部の人々が昼夜を問わず新たな抗vWF VHHsを開発するために努力しています。
このコンセプトは、血友病を持つ人々に相当な貢献をする可能性があります。Anti-vWF VHHsは実験室内で設計されたファームです。これらのタンパク質はvWFに結合し、問題を引き起こすのを防ぎながら、体が不良反応を示すのを避けます。一方で、VHHsは非常に小さく、従来の抗体よりも製造が簡単です。したがって、 三価VHH生産 治療法としてより良い選択肢となるかもしれません。
ヤオハイの科学者たちは、さまざまな手法を使用して、特異的にvWFの機能を妨害する抗vWF VHHを生成しています。 トリスペシフィック VHH の生産 他の血液凝固成分に影響を与えることなく、これを達成するために、時には人間化された特殊なvWFをラマに提供します。その後、これらのVHHはラマの血液から採取されます。ラマは、特定の標的に対して非常に効果的に働く一部のVHHを持つ驚くべき動物です。それは、科学者がVHHを集めた後、それらをさらに改良してより効果的なものにすることができるということを意味します。
科学者たちは別の方法、いわゆるファージディスプレイライブラリーも使用しています。これにより、彼らはバクテリオファージと呼ばれる小さなウイルスに接続されたVHHsのライブラリーを持っています。この方法で、科学者たちはvWFの特定の領域に結合するVHHsを選択することができます。その後、それらを分離して特性を分析します。 Anti-HER2 VHH 生産 その後、これらの薬剤は安全性と効能が評価されます。 Yao Haiチームは他の研究グループや企業とも協力し、追加のVHHを特定し、血友病動物モデルでのその活性を評価しています。この協力関係は、研究の深さを増すために非常に重要です。
最近の研究で、科学者たちは最も強力な抗vWF VHHの一つを特定し、それをVHH-33と名付けました。VHH-33は、vWFによって引き起こされる血小板の凝集を効果的に抑制する能力を持っています。実験室での試験やA型血友病のマウスモデルにおいても、比較的安全に血栓を分解することが示されました――これは治療法をテストする際に期待できるほぼ完璧な成功です。実際に、VHH-33がどのようにしてvWFの働きを止め、その問題を防ぐのかを理解するために、研究者たちはVHH-33をさらに詳しく調べました。
Yaohai BioPharmaは、品質管理と規制事務を統合したトップ10の微生物CDMOです。私たちの品質システムは、現在のGMP基準および国際規制に準拠しています。また、規制の専門家チームは、世界的な規制フレームワークに精通しており、バイオ製品の上市を加速します。私たちはトレーサブルな生産プロセス、品質の高い製品、そしてAnti-vWF VHHの生産やEU EMA、オーストラリアTGA、中国NMPAの要件への適合を確保します。Yaohai BioPharmaは、欧州連合(EU)の資格のある担当者(QP)による対面での監査を無事通過し、私たちのGMPシステムと生産施設が検証されました。さらに、ISO9001品質マネジメントシステムおよびISO14001環境マネジメントシステムの初期認証監査も完了しました。
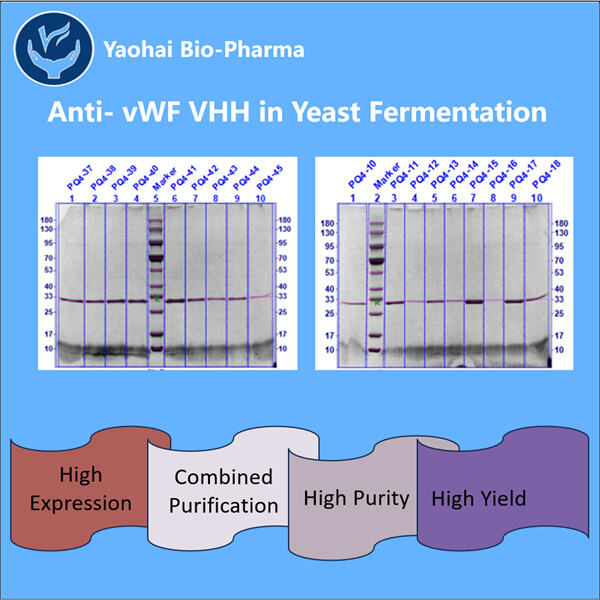
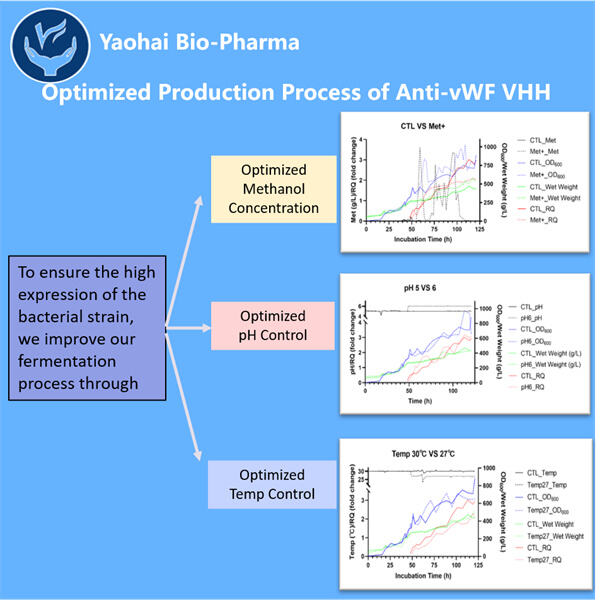
Anti-vWF VHH プロダクションは、微生物発酵に特化したトップ10のバイオテクノロジー企業です。私たちは、強力な研究開発能力と現代的な製造設備を備えた最新鋭の施設を構築しました。GMP基準に準拠した微生物の精製および発酵用に5つの製造ラインと、バイアルやカートリッジ、プリフィルドルーバー用に2つの自動充填・仕上げラインが利用可能です。利用可能な発酵規模は100L、500L、1000L、2000Lです。バイアルの充填仕様は1mlから25mlまで対応しています。プリフィルドシリンジとカートリッジの充填仕様は1-3mlをカバーします。生産用のワークショップはcGMPに準拠しており、商業製品および臨床試験サンプルの安定供給を保証します。私たちの工場では、世界中に出荷される大分子を製造しています。
Yaohai Bio-Pharmaは、微生物バイオ医薬品のリーディングCDMOです。私たちの主な焦点は、ペット、人間、および獣医療の健康を治療するためのAnti-vWF VHHの生産と治療法の開発です。最先端の研究開発プラットフォームと製造技術を持ち、微生物株の開発から細胞バンク、プロセスおよび方法の開発、臨床および商業製造に至るまでの全製造プロセスをカバーしています。これにより、革新的なソリューションの成功裏の提供が保証されます。私たちは時間とともに微生物ベースのバイオプロセッシングに関する広範な知識を得てきました。200以上のグローバルプロジェクトを成功裡に完了し、米国FDA、EU EMA、オーストラリアTGA、中国NMPAの規則や規制に対応するためのお客様のサポートを行っています。私たちの経験と専門知識により、市場のニーズに迅速に反応し、カスタマイズされたCDMOサービスを提供することができます。
ヤオハイ バイオファーマは、微生物由来のバイオ医薬品の開発において豊富な経験を持っています。私たちはカスタマイズされた研究開発および製造ソリューションを提供し、リスクがないことを確実にします。サブユニット型再構成ワクチン、Anti-vWF VHH 生産、サイトカイン、成長因子、単一ドメイン抗体、酵素、プラミドDNA、mRNAなど、多様なモダリティに取り組んできました。また、私たちには様々な微生物に関する専門知識があり、酵母の細胞外分泌および細胞内分泌(最大15g/Lの収量)や、バクテリアの細胞内可溶性および包含体(最大10g/Lの収量)も扱っています。さらに、BSL-2発酵プラットフォームを開発し、バクテリアベースのワクチンを作り出しました。私たちは生産プロセスの改善の実績があり、収量を増やし、コストを削減しています。効率的な技術チームにより、プロジェクトを確実に期限内かつ高品質で納品し、あなたの製品を市場に早く投入します。