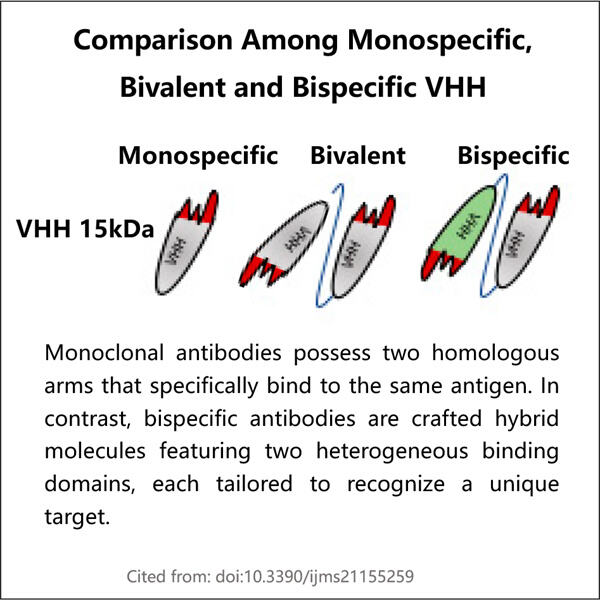
ヤオハイは、人間の生命に必要な栄養素を提供する製品サプライヤーです。彼らが作る独自の薬剤には、ビスペシフィックVHHがあります。では、ビスペシフィックVHHとは何でしょうか?これらは抗体と呼ばれる解剖学的な小さな部分から構築されています。抗体とは、私たちの免疫システムが病気を攻撃し、健康を維持するために使用する小さな戦士のようなものです。ビスペシフィックタイプのVHHは、同時に2つの異なるエンティティに結合できるという点で注目に値します。これは、特定の種類の癌などの一部の疾患の治療において非常に有用です。
これらのビスペシフィックVHHは、ヤオハイが「発現ホスト」と呼ばれる独自の細胞を使用して作成したもので、ヤオハイの製品である 再構成インスリンの製造 ジア。これらの発現ホストは、ヤオハイが薬に使用したい完全に同じ抗体を生成するように訓練されています。バイスペシフィックVHHは、実際には作りにくいです。有时候、細胞が正しく形成されていない抗体を作り出したり、十分な応答がないことがあります。これが理由で、ヤオハイは日夜安全で効率的なバイスペシフィックVHHの製造方法を探しています。
ここに示されているバイオリアクターは、研究者が幹細胞を培養する方法の一つです。バイオリアクターは非常に優れており、一度に多くのビスペシフィックVHHを作ることができます。これは、一度に数個に制限される代わりに、これらのVHHが大量にビスペシフィックに組み立てられ、実質的に無限のビスペシフィックVHH供給が可能になることを意味します。これは時間の節約だけでなく、ヤオハイが新しい薬のテストをできるだけ早く開始することを容易にします。
免疫療法は、ビスペシフィックVHHにとって魅力的な用途の一つを提供します、ちょうど レントウイルスプラミド製造 ヤオハイによって製造されました。免疫療法薬は、あなたの体が病気と戦うのを助ける薬です — 特に癌などのより重度の状態に対して二重特異的に作用します。例えば、二重特異的なVHHは癌細胞と免疫系細胞の両方に結合し、免疫系がこれらの危険な細胞を識別して除去するのを助けます — 免疫療法中に非常に有用です。
しかし、二重特異的なVHHを免疫療法剤として生産することは簡単ではありません。また、ヤオハイの製品である GMP Anti-HER3 VHH 生産 もあります。時々、抗体を作る細胞が疲れてしまい、十分な量のこれらの分子を作ることができなくなります。または、抗体がヤオハイが望むように癌細胞に結合しないことがあります。これが理由で、ヤオハイはこれらの問題を解決するために新しい先進的な方法を探し続けており、良い薬を更新しています。それは癌で苦しんでいる人々のためにです。
これは、患者の血液を抽出して検査し、その人の体に適した完璧な抗体を見つけることで行われます。そして、 GMP Anti-MMRCD206 VHH ヤオハイによって製造されました。そこで、彼らはこれらの専門的な抗体で構成される双特異的VHHを設計します——特にその個人向けに特別に調整されたものです。これによりパーソナライズされた要素が加わり、薬がその特定の個人に効くことをより確実に保証できるようになります。
さらに一つの技術として「アフィニティ成熟」があります。この技術は双特異的VHHを癌細胞にさらに効率的に結合させます。ヤオハイのものと同様です。 VLPベースのワクチン製造 また、双特異的VHHが体内でより長く持続するようにするいくつかの新しい概念も活用しています。免疫システムによって除去されないようにするためです。これは、これらの抗体が活動可能な時間が長いほど(定義上)、病気の予防においてより強力になるため重要です。
Yaohai Bio-Pharmaは、微生物由来のバイオ医薬品の製造において経験を持っています。私たちはリスクを最小限に抑えながら、カスタマイズされた研究開発ソリューションと製造を提供します。我々は、二重特異性VHHの生産、ワクチン(ペプチドを含む)、成長因子、ホルモン、サイトカインなど、多様な方法で取り組んできました。私たちは、酵母の細胞外および細胞内(最大15 g/Lの収量)や、バクテリアのペリプラズム分泌、可溶性細胞内、包含体(最大10 g/Lの収量)など、複数の微生物ホストに特化しています。また、BSL-2発酵プラットフォームを使用してバクテリアワクチンを作り出します。プロセスの改善、製品収量の増加、そして生産コストの削減に特化しています。効率的な技術チームが、プロジェクトのタイムリーかつ高品質な納品を保証します。これにより、あなたの独占製品を市場に迅速に投入することが可能です。
Yaohai BioPharmaは、品質管理と規制事項を組み込んだトップ10の微生物CDMOです。私たちは、現在のGMP基準および国際規制に準拠した品質システムを持っています。当社の規制チームは、グローバルな規制フレームワークに精通しており、バイオ製品の上市を加速させます。また、追跡可能な製造プロセスとトップクラスの品質の製品を提供し、US FDA、EU EMA、オーストラリアTGA、およびビスペシフィックVHH生産の要件に準拠しています。Yaohai BioPharmaは、GMP品質システムと生産サイトについて、ヨーロッパ連合の適格者(QP)による現地審査を成功裏に通過しました。さらに、ISO9001品質マネジメントシステム、ISO14001環境マネジメントシステム、ISO45001職業安全衛生マネジメントシステムの最初の認証審査もクリアしています。
Bispecific VHH Productionは、微生物由来のバイオ医薬品におけるトップクラスのCDMOです。我々の焦点は、ヒト、獣医学、ペット健康管理に適した微生物で生産されるワクチンや治療薬にあります。最先端のR&Dプラットフォームと製造技術を有し、微生物株の開発やセルバンキングからプロセスおよび方法開発、臨床および商業生産までの一連の手順をカバーしています。これにより、革新的なソリューションの成功裏の提供が保証されます。長年にわたり、我々は微生物ベースのバイオプロセッシングに関する広範な知識を蓄積してきました。200以上のプロジェクトを成功裡に完了しており、US FDAやEU EMAなどの規制への準拠を支援します。また、Australia TGAやChina NMPAにも対応します。我々の専門知識と豊富な経験により、市場のニーズに迅速に対応し、カスタマイズされたCDMOサービスを提供することができます。
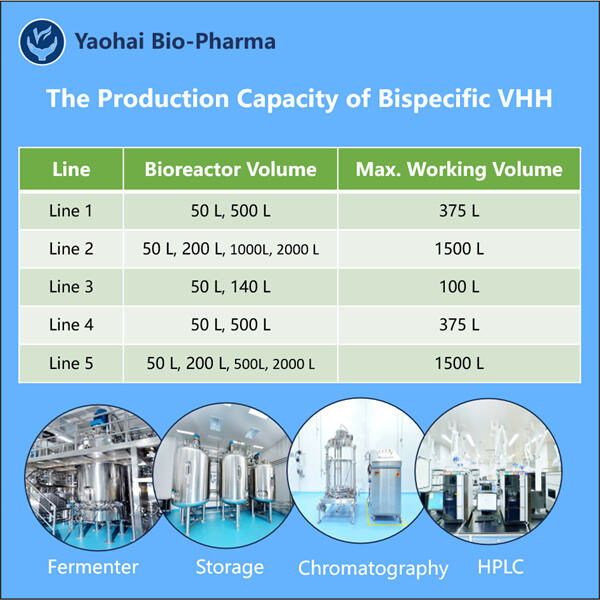
ヤオハイ・バイオファーマは、トップ10のバイオ製品メーカーであり、微生物発酵の専門家です。私たちは、最先端の設備と強力な研究開発および製造能力を備えた施設を構築しました。GMP基準に準拠した5つの医薬品原薬生産ラインがあり、微生物の精製と発酵に対応しています。さらに、バイアル、カートリッジ、プリフィルトシリンジ用の2つの自動充填・仕上げラインも装備されています。利用可能な発酵スケールは100L、500L、1000L、2000Lです。充填容量は1mlからビスペシフィックVHHの生産まで対応しており、プリフィルトのシリンジやカートリッジは1〜3ml相当で充填されます。当社のcGMP準拠の生産工場は、臨床試験サンプルや商業製品の安定供給を確保します。私たちの工場で生産されたバルク分子は世界中へ出荷可能です。