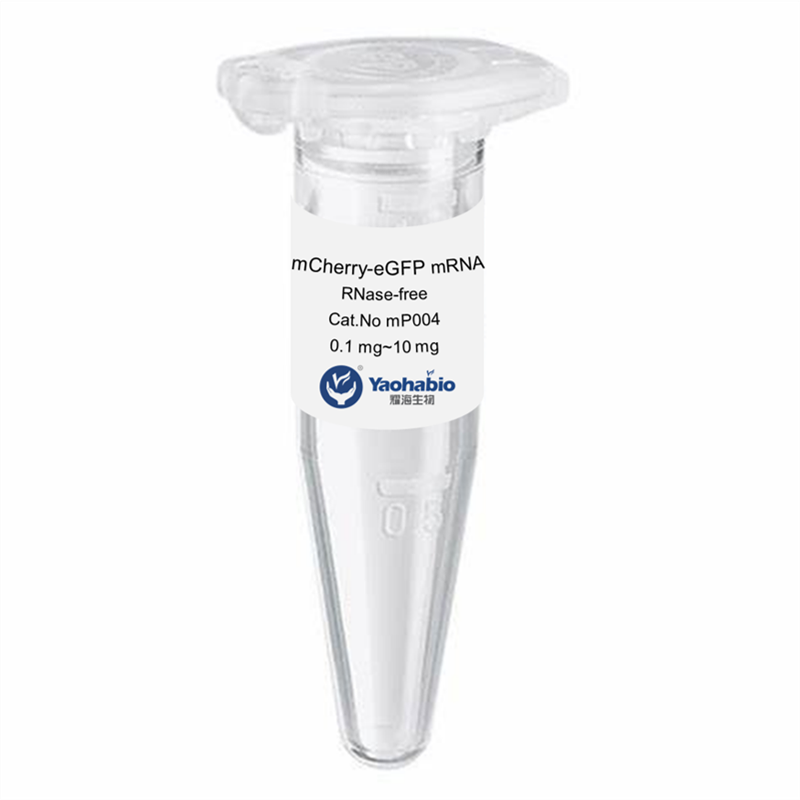
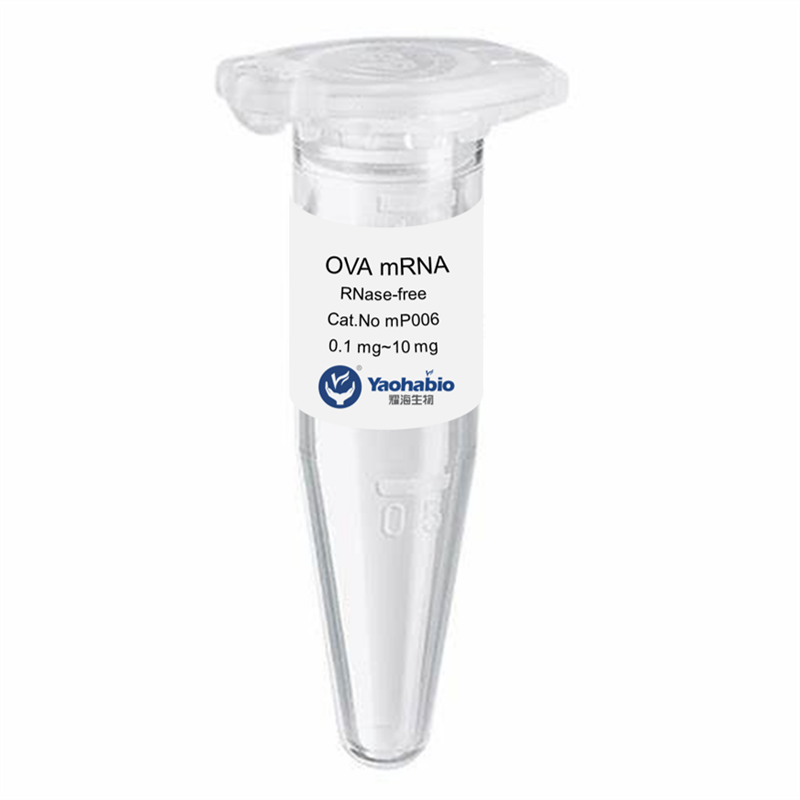
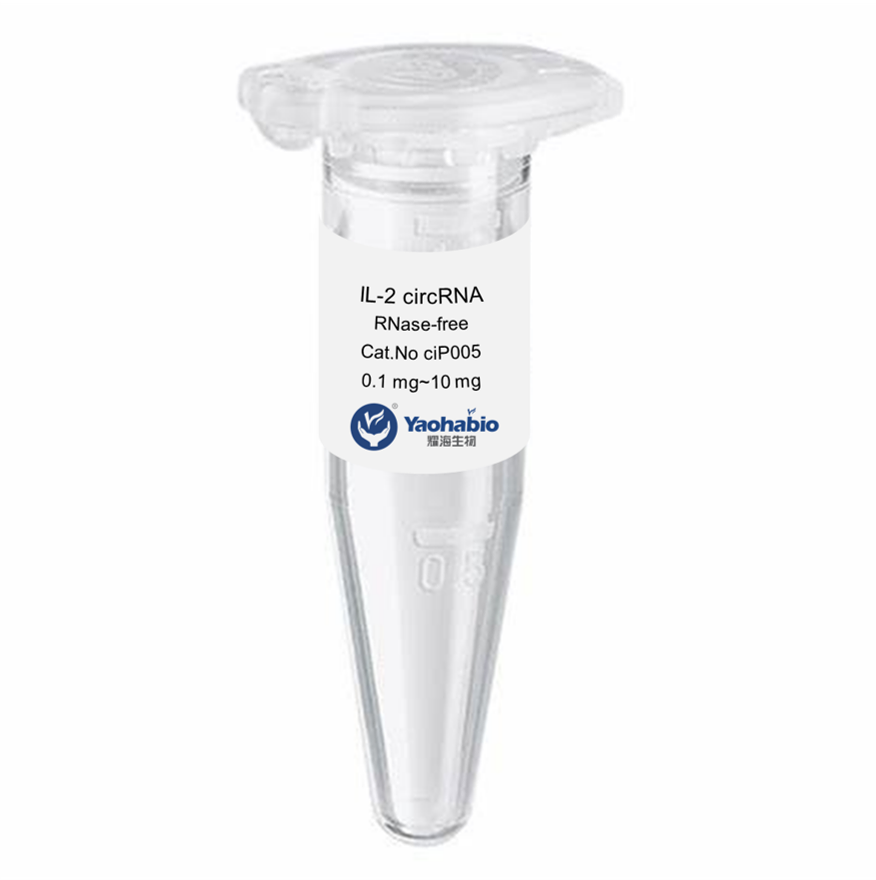
Prilagođavanje, Efikasnost & Kostefikasanost
Yaohai Bio-Pharma ima iskustva u biološkim derivatima iz mikrobioloških izvora. Ponudamo prilagođene R i D rješenja te usluge proizvodnje, minimizirajući potencijalne rizike. Bili smo angažirani u brojnim modalitetima poput podjediničnih cjepiva, rekombinantnih peptida, hormona, citoquina, rasta faktora, jednodomenskih antigena, enzima, plazmidnog DNA, mRNA-a i drugih. Specializirali smo se na nekoliko mikroorganizama poput kvasca, ekstracelularno i intracelularno GMP GLP-1 Proizvodnja (dohodak do 15g/L) i intracelularno rastuće bakterije te inkluzijska tijela (dohodak do 10g/L). Također imamo BSL-2 fermentacijsku platformu za stvaranje bakterijskih cjepiva. Fokusiramo se na optimizaciju procesa, povećanje dobivenih proizvoda i smanjenje troškova. Imamo učinkovit tim stručnjaka koji osigurava vremeno i visokokvalitetno dostavljanje projekata. To nam pomaže donijeti vaše jedinstvene proizvode brže na tržište.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN