Uudistuneen HSA:n sovellukset lääketieteellisessä tutkimuksessa ja hoitoprojekteissa
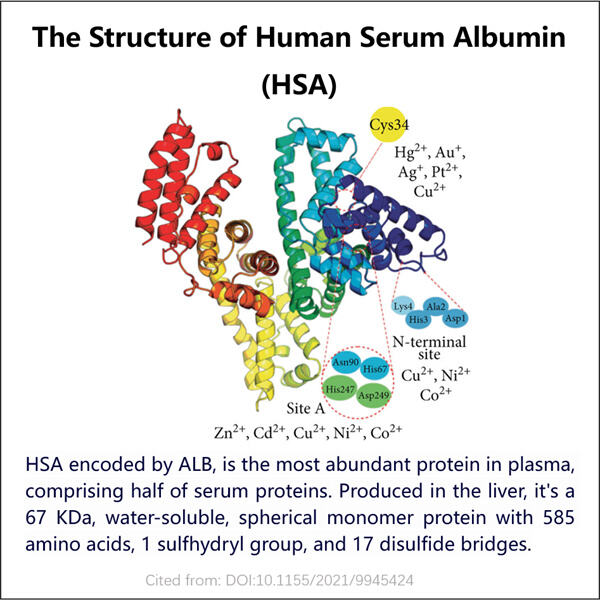
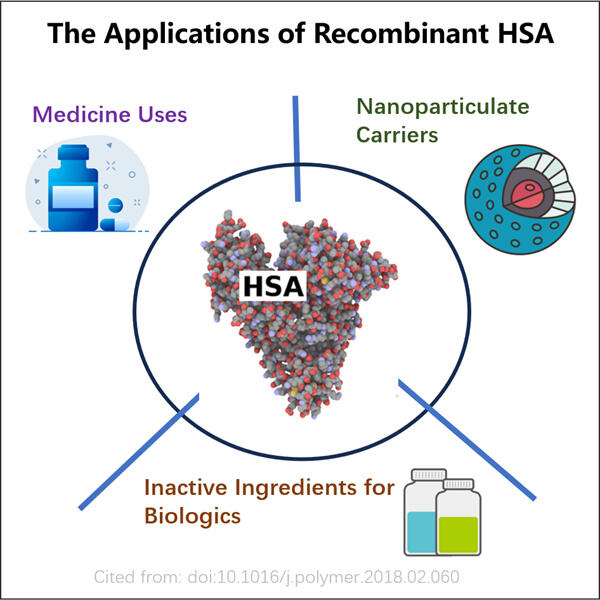
Ihmisen verenserumalbumi (HSA) on mukana monissa eri osa-alueissa lääketieteen tutkimuksessa ja hoitotapoissa, ja koska se on suhteellisesti hyvin tutkittu biologinen molekyyli, geeni, joka koodaa HSA:ta, on rekoombinoitu erilaisiin iskupohjoihin helpottaakseen sen puhdistusta. Tämä voi olla hyödyllistä esimerkiksi siirtääksesi lääkettä tehokkaammin jonkin elimen tiettyyn alueeseen. Se on erittäin kriittistä, koska se antaa vain yhden paikan, jonne lääke voi liikkua tarvittaessa. GMP Anti-MMRCD206 VHH voidaan myös käyttää näytteenoton tarkoituksena, katsoa piilottavatko maksamme niin paljon HSA:a uriniin, että tämä testi tulee positiiviseksi. Yksityiskohdat voivat auttaa lääkäreitä saamaan lisätietoja yhden henkilön terveydestä ja päättämään, mikä olisi oikea lähestymistapa potilaanhoidossa.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN