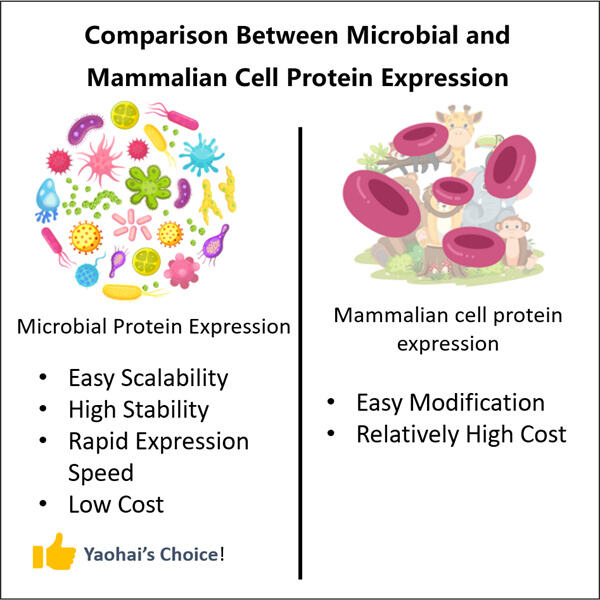
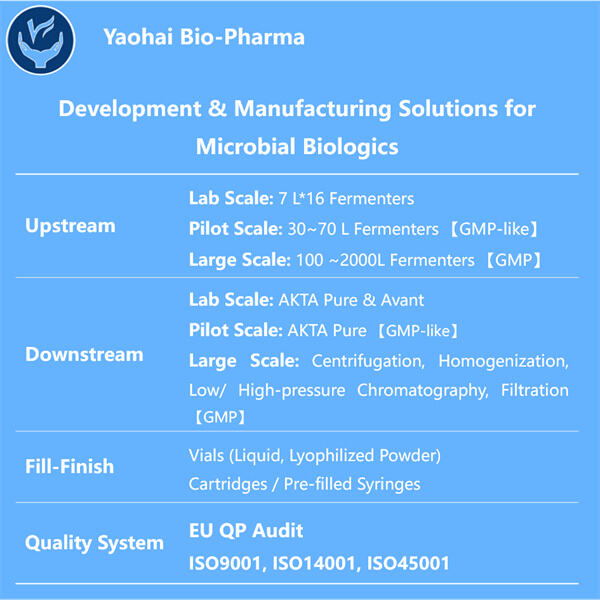
Yaohai GMP Výroba Anti-CD8 VHH je naozaj vzrušujúce zdieľať s vami najfreššie informácie o mikrobiálnych biologických liečstvách! Vieťe, môže to byť slovo veľmi veľké, ale nie je tak zložité, ako sa zdá. Mikrobiálne biologické liečivá: Živé entitety na malom mierku, ako sú baktéri a húbky, sú nevyhnutné na pomoc pri vyvíjaní liečiv. Tento príspevok vysvetlí, čo sú mikrobiálne biologické liečivá, ich produkcia a výroba a prečo sú dôležité pre zdravie a medicínu.
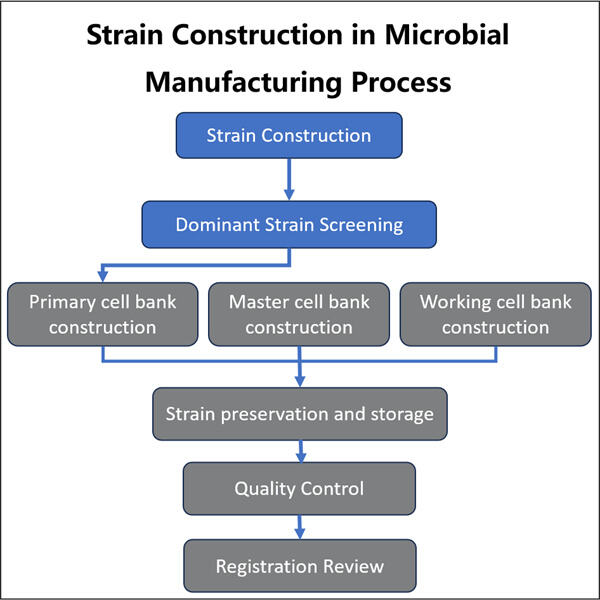
Máme mnoho nových a cool nástrojov, ktoré môžeme použiť na výrobu mikrobiálnych biologických liečív. Jednou z nových metód je fermentácia. Pri fermentácii (rastu malých živých organizmov ako sú baktérie alebo kvasinky v veľkých nádobách nazývaných bioreaktory) krmením týchto mikroorganizmov v bioreaktoroch, čo im umožňuje dýchať a rásť. Z niekoľkých malých buniek sa vyvinú na desiatky miliónov. Ak máme dostatočný počet mikroorganizmov, môžeme ich potom získať - alebo sklístať ich a vyrobiť veľmi dôležité liečivá.

 SK
SK
 EN
EN AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN