With the widespread application of mRNA COVID-19 vaccines in large populations, the safety of mRNA vaccines has been validated. mRNA possesses the ability to express any protein, offering potential solutions to various unmet clinical needs.
Yaohai Bio-Pharma provides a comprehensive solution for mRNA R&D and GMP production, backed by a robust research platform and a compliant GMP system. Our services are tailored to meet the unique requirements of our clients, offering them high-quality mRNA drug substances and LNP-mRNA finished products in quantities from milligram to gram, as well as detailed development and production reports, and testing reports.
We have obtained authorization for LNP patent technology from our partner, NanoStar Pharmaceuticals, ensuring to avoid potential patent disputes in the future.
mRNA/LNP One-Stop Solution of Yaohai Bio-Pharma
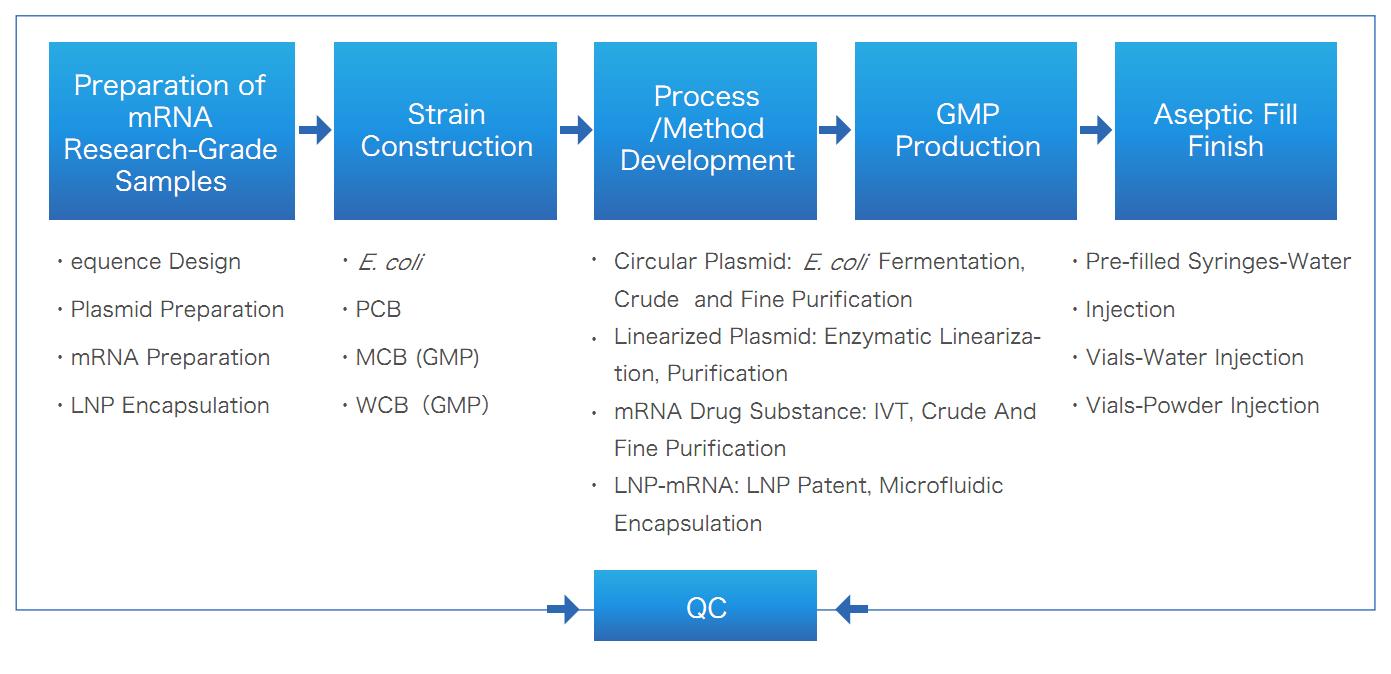
Deliverables
| Grade |
Deliverables |
Specification |
Applications |
| non-GMP |
Drug Substance, mRNA |
0.1~10 mg (mRNA) |
Preclinical research such as cell transfection, Analytical method development, Pre-stability studies, Formulation development |
| Drug Product, LNP-mRNA |
| GMP, Sterility |
Drug Substance, mRNA |
10 mg~70 g |
Investigational new drug (IND),Clinical trial authorisation (CTA), Clinical trial supply, Biologic license application (BLA),Commercial supply |
| Drug Product, LNP-mRNA |
5000 vials or pre-filled syringes/cartridges |
Yaohai’s mRNA CRDMO service, covering the entire life cycle of mRNA
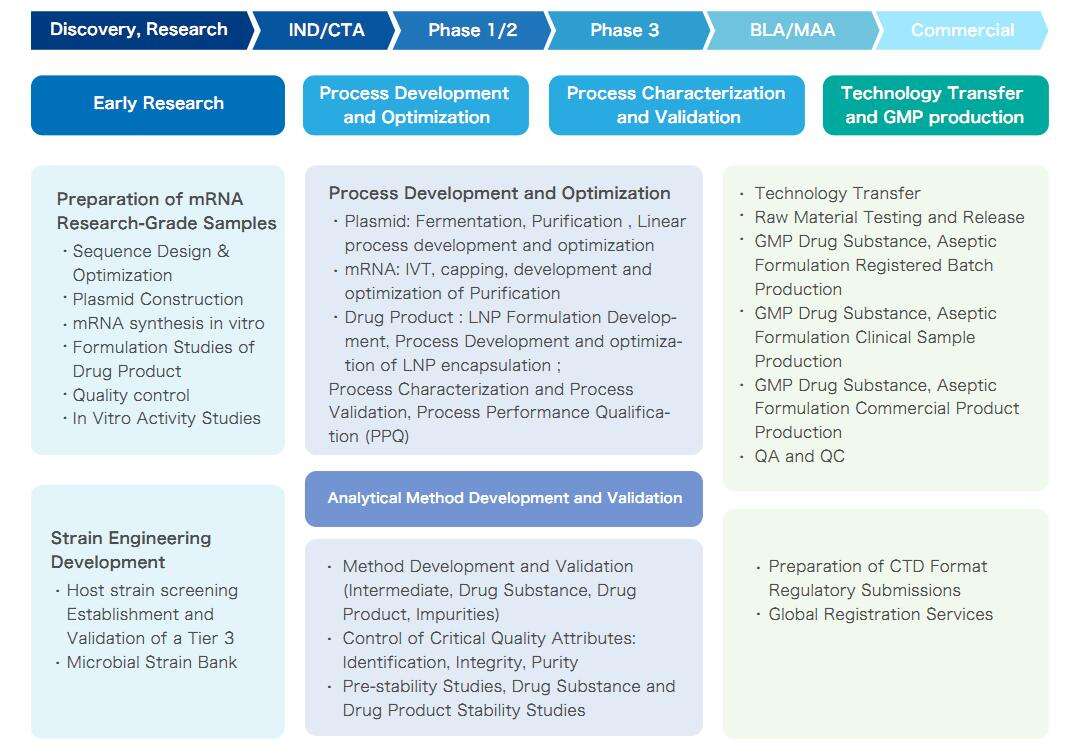
Platform Features
Plasmid DNA Platform
- Multiple 7L fermentation systems, animal-free throughout the process
- Clear traceability of plasmids and host bacteria, with no declaration obstacles
- The yield of Plasmid containing poly A exceeding 500 mg/L
- Poly A loss rate less than 5 bp
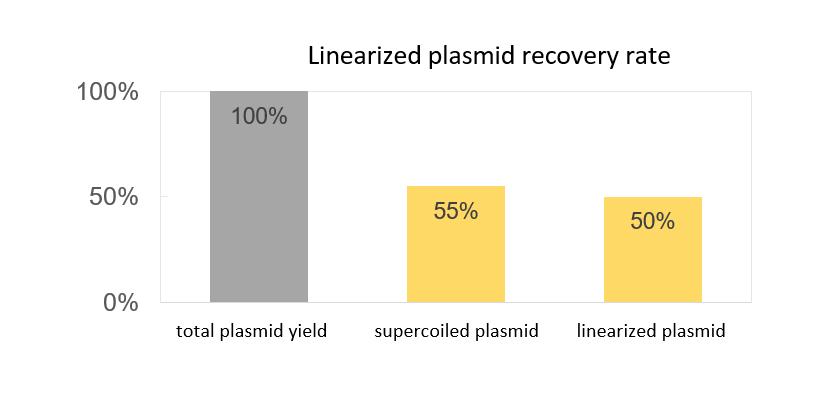
- Supercoiled plasmid proportion greater than 90%; recovery rate over 55%
- Linearization efficiency exceeding 99%; linearized plasmid recovery rate of 90%
Drug substance Platform of mRNA
- Multiple 1L Reactors (GMP)
- A high transcription ratio of 1: 120, allows for a scalable IVT process
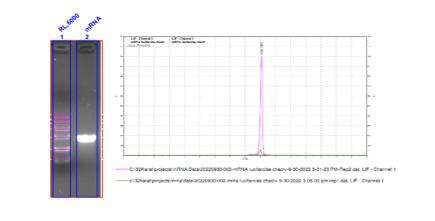
- mRNA integrity exceeding 98%
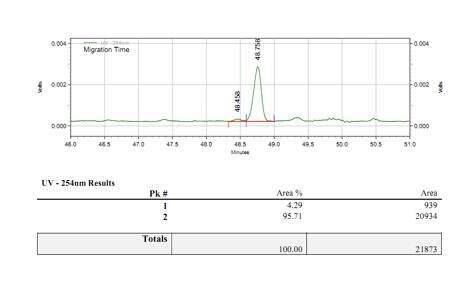
- Stable capping process with a capping rate of over 95%
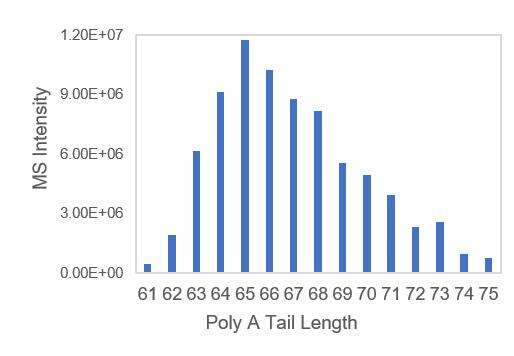
- Transcription templates with A-tails, ensuring uniform distribution of poly A tails.
LNP Encapsulation Platform
- LNP patent technology authorized by our partners to ensure avoidance of patent disputes for our customers.



(Our partners)
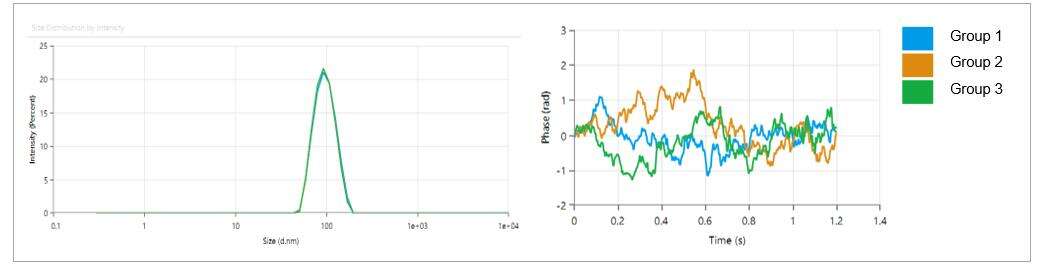
- Employing a highly versatile microfluidic encapsulation process, achieving an encapsulation efficiency of over 95%.
- LNP particle size is within 80-100 nm, with a low polydispersity index (PDI) of 0.05, indicating a uniform distribution of particle sizes.
- LNP particles exhibit a weak charge, with a Zeta potential of approximately -2.18 mV.
| Testing Item |
Testing Method |
Testing Result |
| Encapsulation Efficiency |
Ribogreen |
92.7% |
| Particle Size |
Malvern |
92.07 nm |
| PDI |
Malvern |
0.05 |
| Zeta |
Malvern |
-2.18 mV |
Method Development Platform
We offer a comprehensive method development platform for analyzing circular and linearized plasmids, mRNA raw materials, and finished LNP-mRNA products. Our analysis covers a variety of parameters, such as integrity, purity, capping efficiency, poly A distribution, encapsulation efficiency, particle size, LNP components, and various process residuals (HCP, HCD, HCR, dsRNA, antibiotics, DNase I, T7 RNA polymerase, vaccinia capping enzyme, 2-O methyltransferase, etc.).
Partial methods are demonstrated as follows:
Detection of mRNA Integrity (Capillary Electrophoresis)
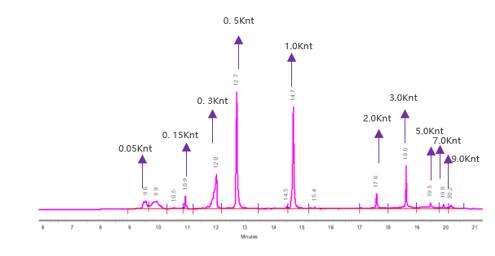
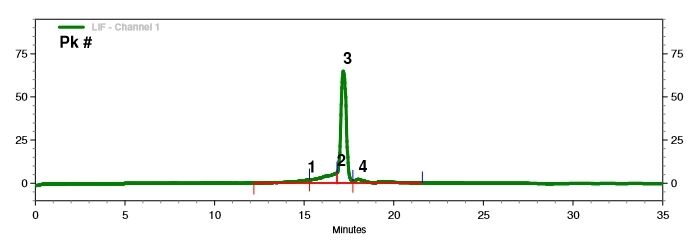
We have developed optimal separation conditions to precisely separate mRNA molecules of varying lengths.
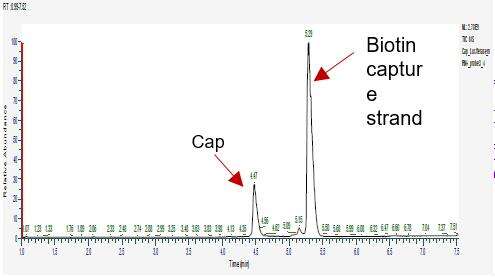
Detection of mRNA Capping Efficiency (LC-MS)
We have developed suitable conditions for 5' end cleavage and separation of 5' end oligonucleotides, allowing for accurate separation of capped and uncapped fragments.
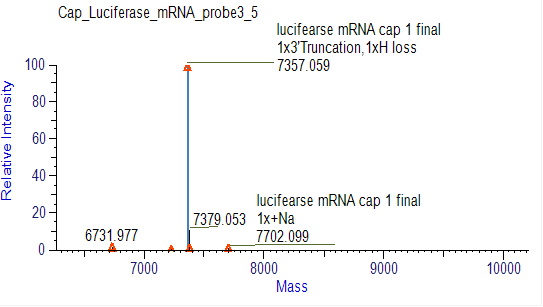
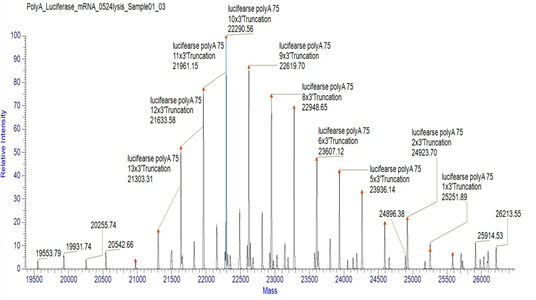
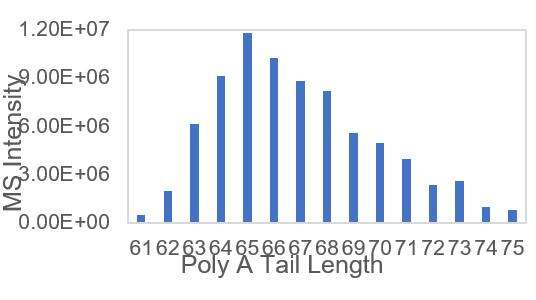
Detection of mRNA PolyA suitable Tail Distribution(LC-MS)
We have developed suitable conditions for the cleavage of 3' ends and separation of 3' end oligonucleotides, which enable precise detection of the distribution of polyA tails.
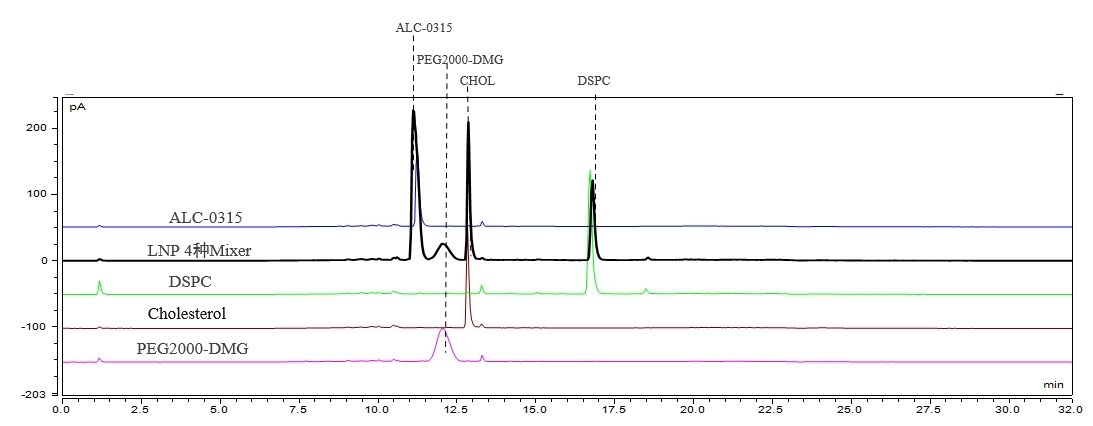
LNP Component and Content Detection (HPLC-CAD)
We have established a suitable chromatographic method that achieves baseline separation of four LNP components. This method demonstrates excellent reproducibility.
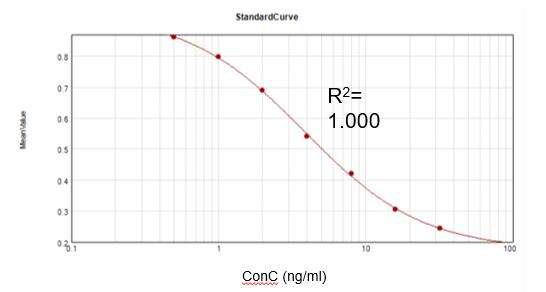
Residual Kanamycin Concentration(ELISA)
Based on a commercial assay kit, we obtained a suitable calibration curve (R2 = 1.000) and achieved a recovery rate of 104.8%.
Residual dsRNA Concentration
Based on a commercial assay kit, we obtained a suitable fitting calibration curve (R2 = 0.999) and achieved a recovery rate of 105.5%.
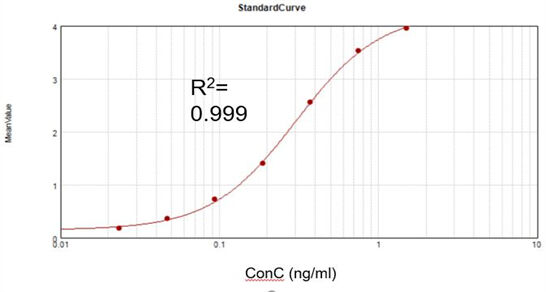
Residual T7 RNA Polymerase (Elisa)
Based on a commercial assay kit, we obtained a suitable fitting calibration curve (R2 = 1.000) and achieved a recovery rate of 107.9%.
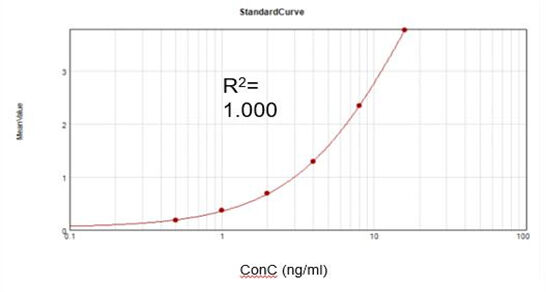
Residual Vaccinia Virus Capping Enzyme (ELISA)
Based on a commercial assay kit, we obtained a suitable fitting calibration curve (R2 = 1.000) and achieved a recovery rate of 92%.
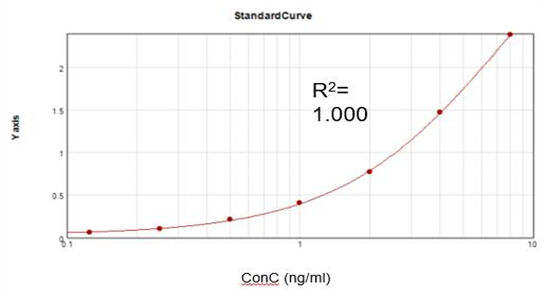

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN