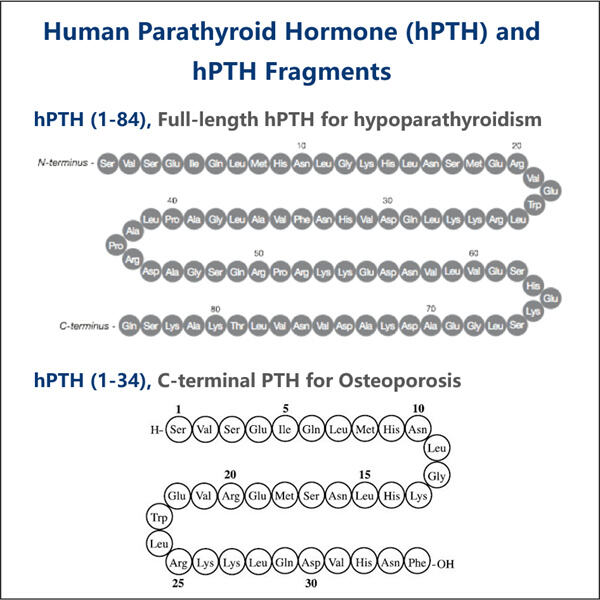
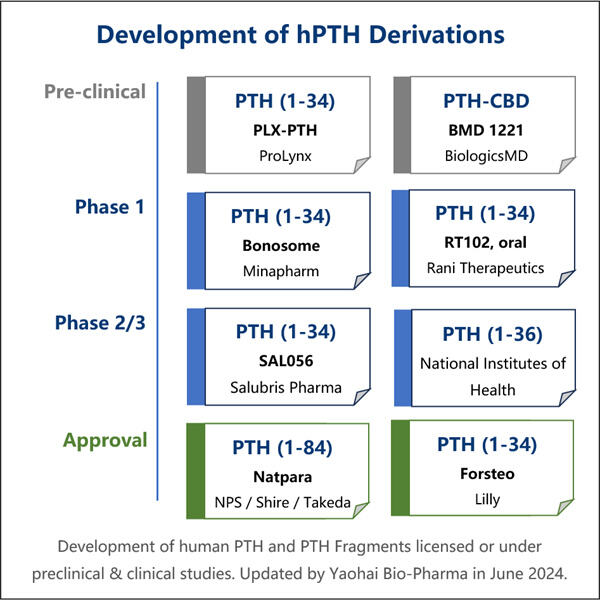
O processo por trás da produção de PTH recombinante
Para fazer as bactérias cozinharem, eles primeiro preparam um pouco de DNA (o livro de receitas mágico que diz aos pequenos insetos quando e onde ligar). Então, eles colocam esse DNA em bactérias. Então eles cultivam essas bactérias em um laboratório. Yaohai Fabricação de peptídeos recombinantes GMP libera o hormônio PTH, que faz com que eles se reproduzam como nós, e produzam mais PTH. Então a alquimia é uma maneira eficaz de obter muito desse hormônio.
Supine PTH Productions Além disso, este recurso também ajuda a produzir técnicas muito mais econômicas com qualidade de Itens de PTH para os consumidores. O PTH é produzido por métodos convencionais que são caros, pois são baseados em PTH animal e humano. Esta pode ser uma abordagem demorada e que exige muitos recursos, principalmente quando você precisa sintetizar grandes quantidades do hormônio.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NÃO
NÃO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN