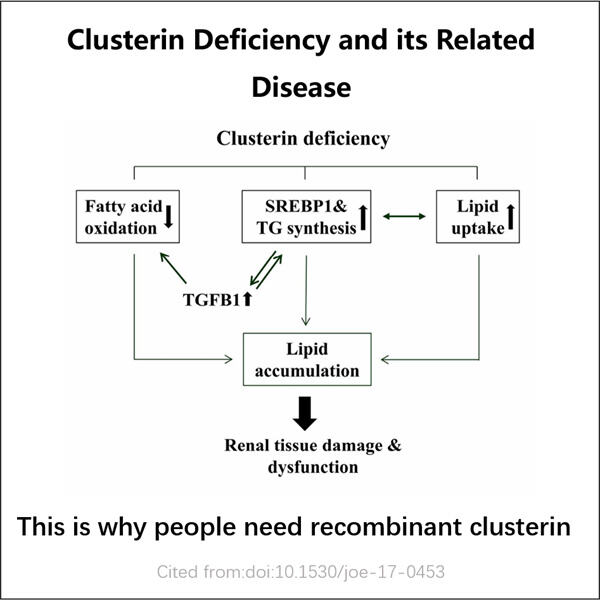
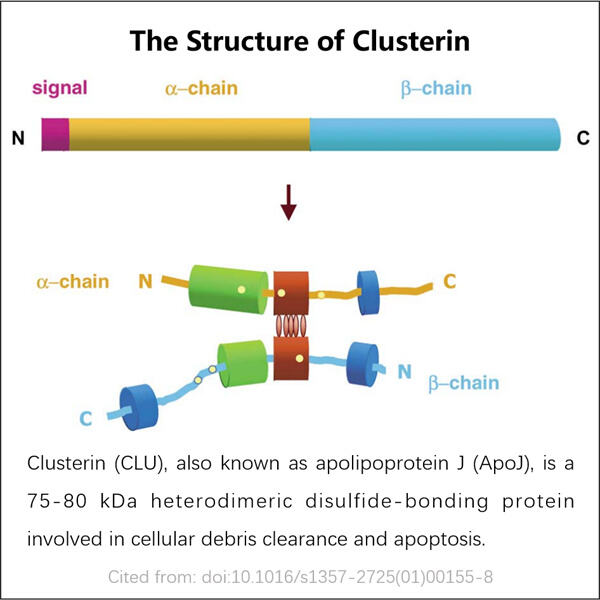
Přizpůsobení, efektivita a hospodárnost
Yaohai Bio-Pharma má zkušenosti s vývojem mikrobiálních biologických látek. Nabízíme přizpůsobené RD i výrobní řešení, přičemž se ujišťujeme, že neexistují žádná rizika. Pracovali jsme na různých modalitách, jako jsou rekombinantní vakcíny na bázi podjednotek, výroba rekombinantních klastrů (CLU), cytokiny, růstové faktory, jednodoménové protilátky, enzymy, plazmidová DNA, mRNA a další. Jsme odborníky na různé mikroorganismy, včetně kvasinkové extracelulární a intracelulární sekrece (výtěžky až 15 g/l) a také na bakterie intracelulárně rozpustné a inkluzní tělíska (výtěžky až 10 g/l). Vyvinuli jsme také fermentační platformu BSL-2 pro vytvoření vakcín na bázi bakterií. Máme zkušenosti se zlepšováním výrobních procesů, a tím i zvyšováním výnosů a snižováním nákladů. S vysoce efektivním technologickým týmem zajistíme včasné a kvalitní dodání projektů a uvedeme vaše produkty na trh rychleji.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NE
NE
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN