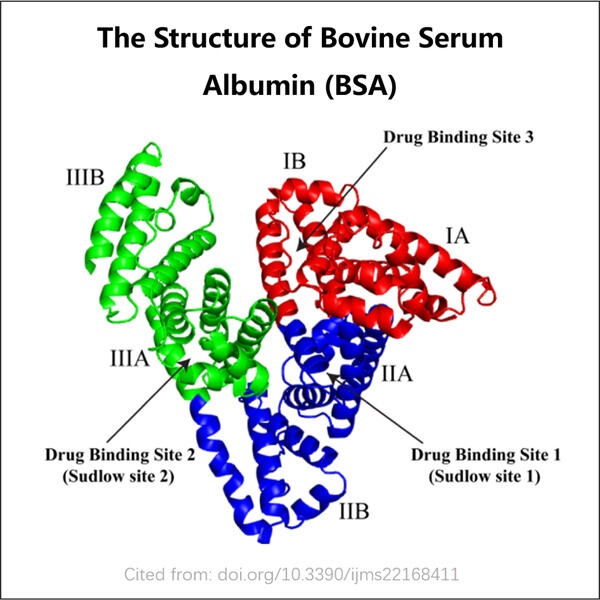
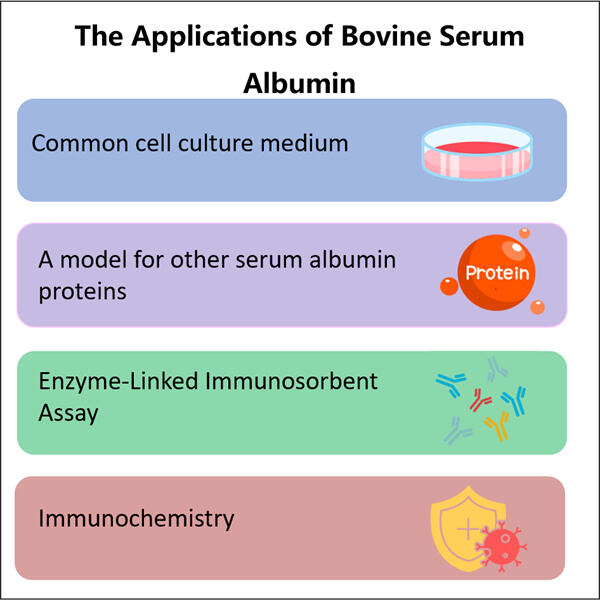
'n Tipe eiwit, bekend as Yaohai Recombinant Bovine Serum Albumin en wat ons algemeen as rBSA verwys, is hoogs beduidend in sowel geneeskunde as wetenskap. Hierdie eiwit word in 'n laboratorium geproduseer deur middel van rekombinerende DNA tegnieke. Met ander woorde, wetenskaplikes gebruik beesteede DNA om die beeste te leer hoe om hierdie eiwit op 'n veilige en gecontroleerde manier te produseer.
'n Groot deel van die rede waarom rekonstitueerde BSA essensiële word, is dat dit veral veiliger en baie koste-effektiefer is wanneer dit vergelyk word met normale BSA wat afkomstig is van beesteeiwit. Tipies Herstelende Hirudin Vervaardiging vervoer af en toe onweerstaanbare siektes, soos volslagtiekte by runder, wat menslike wees baie ernstig kan raak. Verder wissel die kwaliteit van gewone BSA tussen verskillende bronne. Dit maak dit moeilik vir wetenskaplikes en mediese professionele wat betroubare resultate wil hê. In teenstelling daarmee word rBSA in 'n gekontroleerde laboratoriumomgewing vervaardig, wat dit veel meer konsekwent en veiliger maak.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN