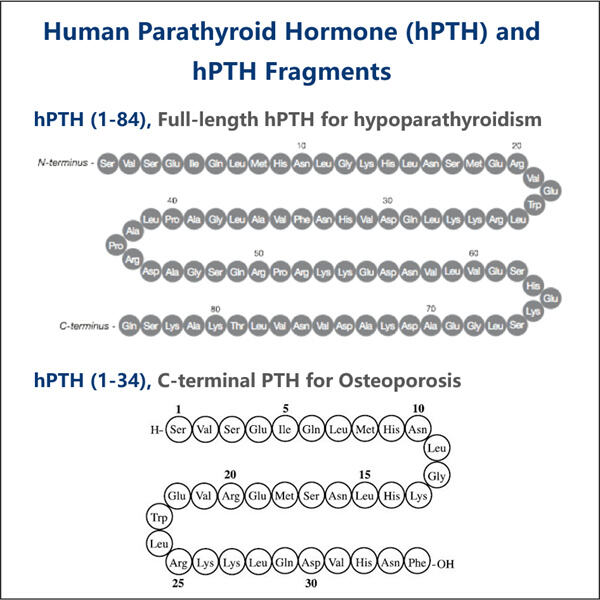
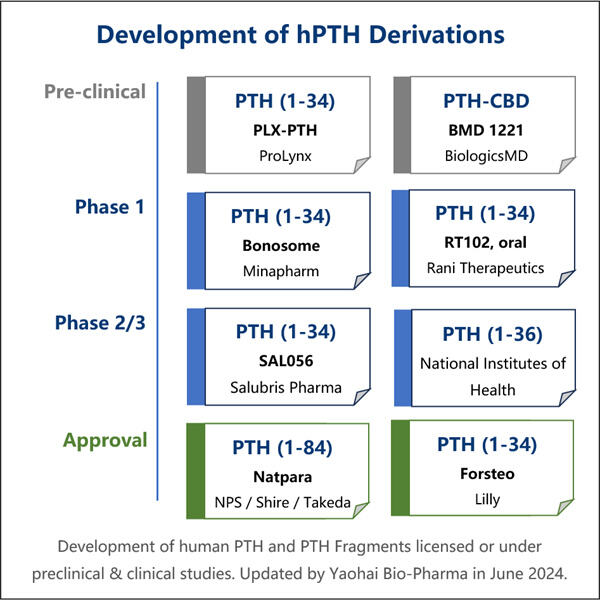
The Process Behind Recombinant PTH Production
Bakterileri pişirmek için önce biraz DNA (küçük yaratıklara ne zaman ve nerede açılacağını söyleyen sihirli yemek kitabı) hazırlıyorlar. Sonra bu DNA'yı bakterilere yerleştiriyorlar. Sonra bu bakterileri bir laboratuvarda büyütüyorlar. Yaohai GMP Rekombinant Peptit Üretimi releases the PTH hormone, which causes them to reproduce just like we do, and make more PTH. So alchemy is an effective way to get lots of this hormone.
Supine PTH Productions On top of that, this resource additionally aids to produce a lot more budget-friendly the techniques with PTH Items quality for consumers. PTH is produced through conventional methods that are expensive as they are based on animals and human PTH. This can be a time-consuming and resource-intensive approach, particularly when you have to synthesize large quantities of the hormone.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 YOK HAYIR
YOK HAYIR
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN