Personalizzazione, Efficienza e Costi Contenuti
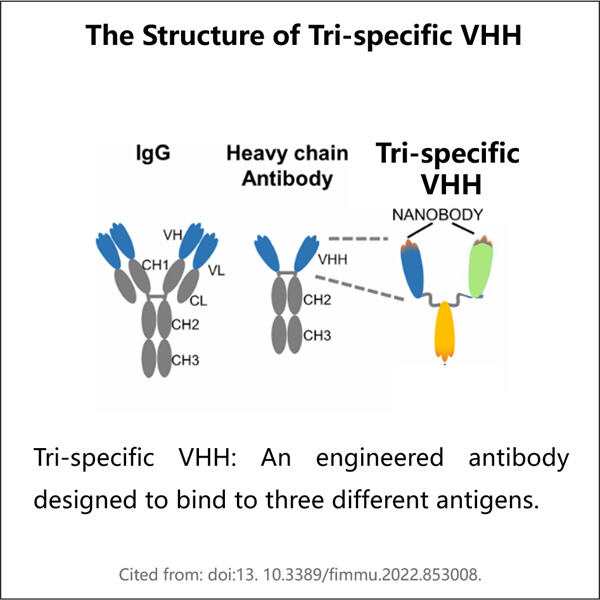
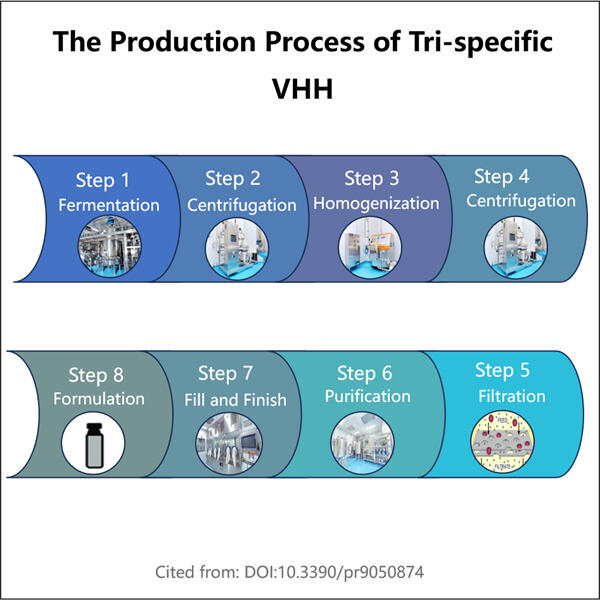
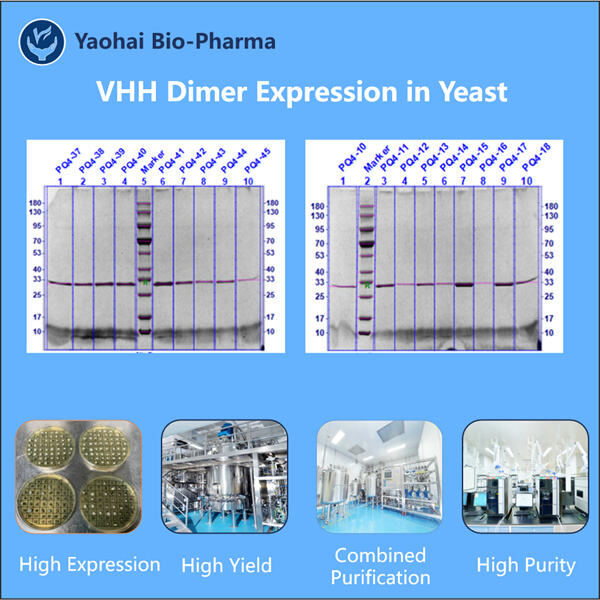
Yaohai Bio-Pharma ha esperienza nella produzione di biologici creati a partire da microorganismi. Offriamo soluzioni su misura per la Ricerca e lo Sviluppo, nonché servizi di produzione, minimizzando i potenziali rischi. Abbiamo lavorato con tecniche diverse, come sottounità cellulari ricombinanti, vaccini (compresi i peptidi), fattori di crescita, ormoni e la produzione Tri-specifica VHH. Siamo specialisti in molti microorganismi, come segretione extracellulare ed intracellulare di lieviti (rendimenti fino a 15g/L) e solubilità intracellulare batterica, e corpo di inclusione (rendimenti fino a 10g/L). Abbiamo anche sviluppato una piattaforma di fermentazione BSL-2 per creare vaccini batterici. Abbiamo un curriculum di miglioramento dei processi produttivi, aumentando i rendimenti e riducendo i costi. Abbiamo un team tecnologico altamente efficiente che garantisce la consegna tempestiva e di alta qualità dei progetti. Ciò ci aiuta a portare i vostri prodotti unici più velocemente sul mercato.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN