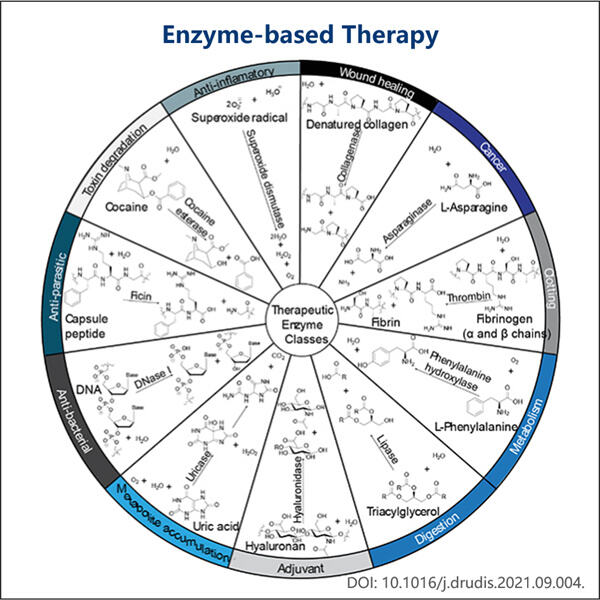
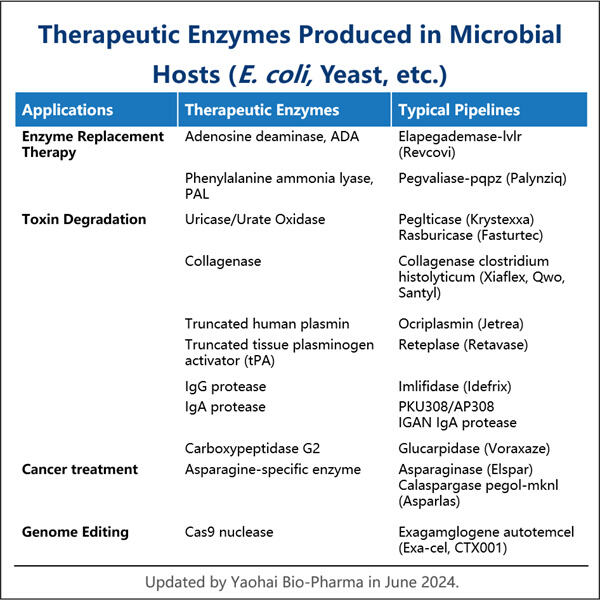
Enzymes are special molecules which help chemical reactions happen in our bodies — an understanding that scientists have only come to appreciate since the early 20th century. Not only are these enzymes critical for everyday functioning, but they can also be harnessed for the treatment of a variety of diseases — which is pretty cool! The biotech company Yaohai is focusing its efforts on engineering new enzyme therapies aimed at an array of health problems.
Some of the enzymes are already used as therapeutics. For example, they help dissolve clots in the blood during a heart attack, which is potentially life-threatening. The research team is currently investigating the potential for enzymes to be used to treat different forms of cancer or inherited diseases. At one end of the spectrum, these diseases are very challenging to treat and this is an important thing. Enzymes can be custom-designed to attack virtually any cell or protein within the body, which makes them efficient for treating most of the illnesses. The ability to address particular regions makes enzyme treatments also safer and associated with fewer side effects than traditional drugs.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 EI
EI
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN