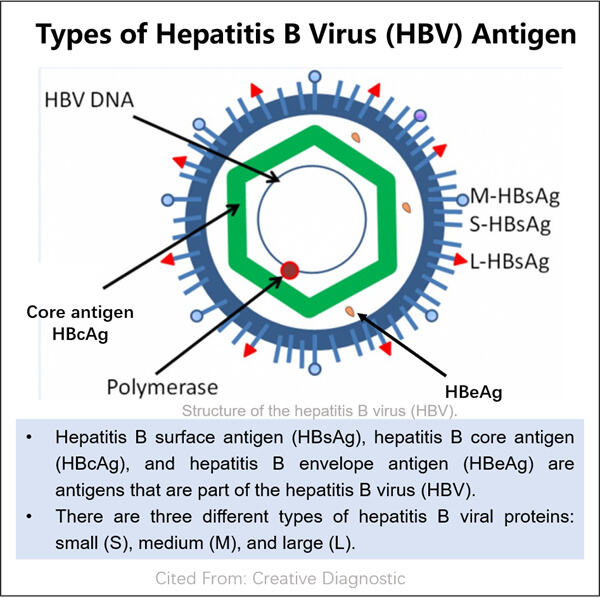
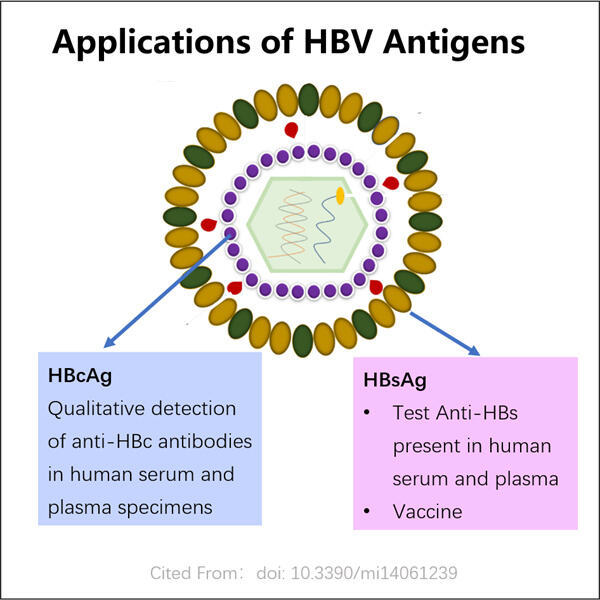
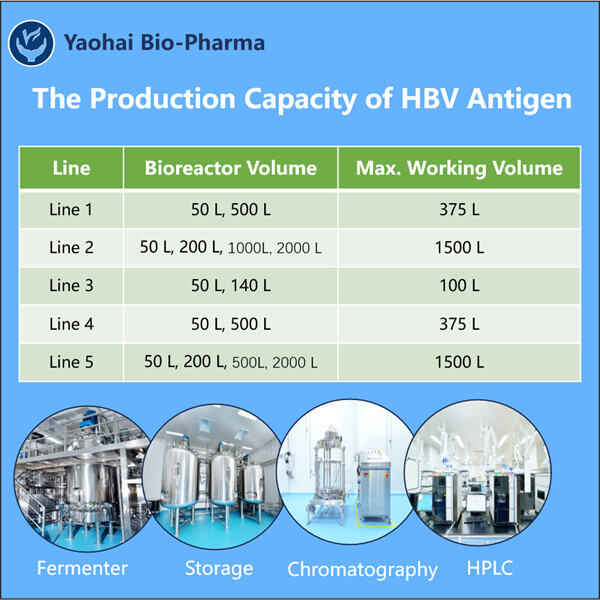
Da, Yaohai este o companie care inovează cu dezvoltarea de diagnosticare a bolilor, precum și strategii de tratament (inclusiv medicina personalizată) pentru acestea. Există o boală cunoscută sub numele de virusul hepatitei B sau HBV. În primul rând, pentru a înțelege mecanismul cu care ajută Producție VHH anti-vWF, trebuie să știm ce este VHB și ce face în interiorul corpului nostru?
Hepatita B (VHB) este un virus care dăunează ficatului. Ficatul este un organ esențial din corpul nostru care participă la digestia alimentelor și la eliminarea substanțelor nocive. O singură persoană atinge sângele sau fluidele corporale ale unei persoane infectate și ajunge să fie infectată cu acest virus care se răspândește atât de ușor. Dacă cineva are HBV, virusul crește și se înmulțește în celulele hepatice. Produce antigene pe măsură ce se replic. Antigenele sunt de fapt proteine, așa că sunt destul de unice pentru a fi cunoscute în sânge.

 RO
RO
 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NU
NU
 PL
PL
 PT
PT
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN