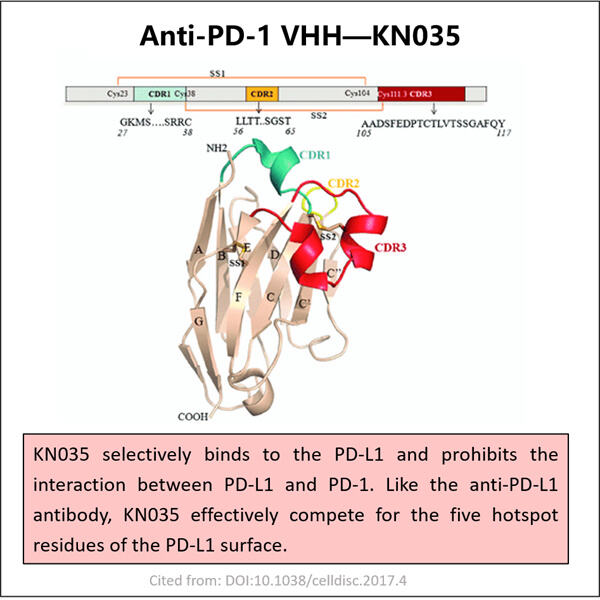
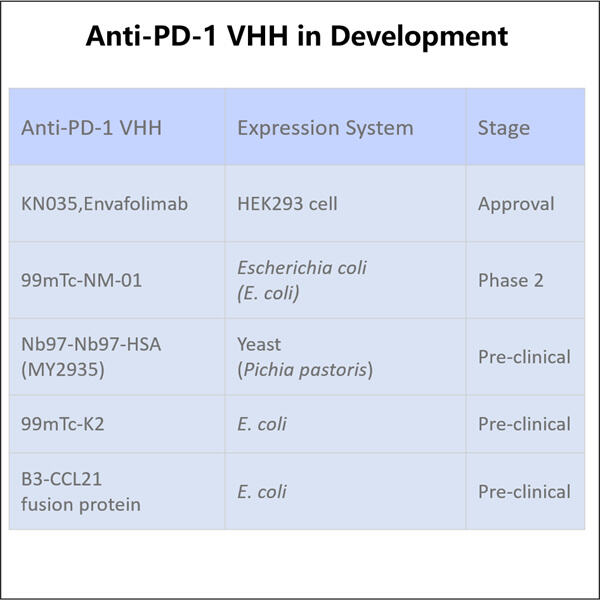
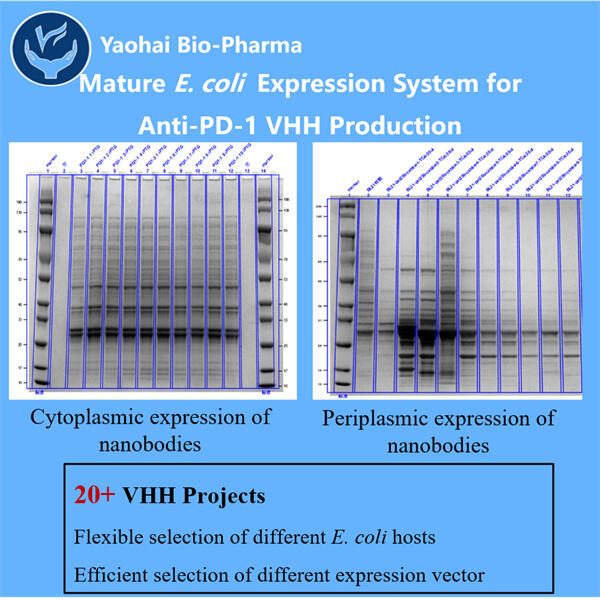
Personalizare, Eficiență și Costuri Reduse
Yaohai Bio-Pharma are experiență în fabricarea de biologice create din microorganisme. Ofertăm soluții RD personalizate precum și servicii de producție, minimizând riscurile. Am colaborat cu diverse modalități, cum ar fi vaccinuri subunitare recombinante, peptide, hormoni, citocine, factori de creștere, anticorpi mono-domeniu, enzyme, ADN plasmid, mRNA și altele. Ne-am specializat în mai multe microorganisme, cum ar fi levurii cu secreție extracelulară și intracelulară (rendiment până la 15g/L) și bacterii cu solubilitate intracelulară și corpuri de incluziune (rendiment până la 10g/L). Am creat de asemenea un sistem de fermentație BSL-2 pentru a produce vaccinuri GMP Anti-PD-1/PD-L1 VHH. Suntem experți în optimizarea proceselor de producție, creșterea rendimentului și reducerea costurilor. Avem o echipă tehnică extrem de eficientă care asigură livrarea proiectelor în timp și cu cea mai bună calitate. Acest lucru ne permite să aducem produsele dvs. unice mai repede pe piață.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN