バイオロジクスは、細胞、タンパク質、または動物や植物の一部を使用して作られる薬剤の一種です。これらの薬剤は、多くの疾患を診断し、治療するために重要です。その他の疾患には、軽度から重度、さらには癌や自己免疫疾患、患者数が非常に少ない希少遺伝的障害なども含まれます。これらに対してもヤオハイは 製品 必要に応じた組織によって安全性と効果が確認された場合にのみ、患者向けに販売されることがあります。アメリカのFDAとヨーロッパの欧州医薬品庁(EMA)などがあります。
バイオ製剤を承認を得るには非常に難しい、複雑なプロセスがあります。例えば、ヤオハイにはこの厳しいプロセスを理解し、適切に進行させる専門家がいます。ヤオハイ バイオロジクス分析法の開発 彼らの責任は、すべてのバイオ医薬品が安全であり、期待される作用を発揮することを確実にすることです。このチームはFDAやその他の規制機関と連携し、求められる要件について議論します。そして、医薬品の承認プロセスを裏付けるために必要なすべてのデータや情報を収集し、含めます。
販売承認を得た後も、単にそのバイオ医薬品を売るだけでは済みません。医薬品は厳格な規制のもとで製造され、販売される必要があります。これらは妖海が順守するべき規則です。 バイオ医薬品の精製プロセス開発 これらのルールは、すべての個々のバイオ医薬品が安全に製造され、常に高品質基準を満たすことを保証するために存在します。妖海は非常にコンプライアンス重視の企業であり、これらの同じルールに従うことを目指しています。このようにして、彼らはすべてのバイオ医薬品アイテムが効果的に作られ、広告されることを確実にすることができます。
しかし、バイオ医薬品は特定の複雑なケアを必要とする患者にとって大きな可能性を秘めています。これは、特定の疾患や状態に関連する分子を標的とした薬を作ることを意味します――つまり、さまざまな健康問題に対して多くの画期的な治療法となる可能性があるということです。ヤオハイはバイオ医薬品の価値を認識し、未充足の医療ニーズに対応する新しい革新的な医薬品を提供することに尽力しています。
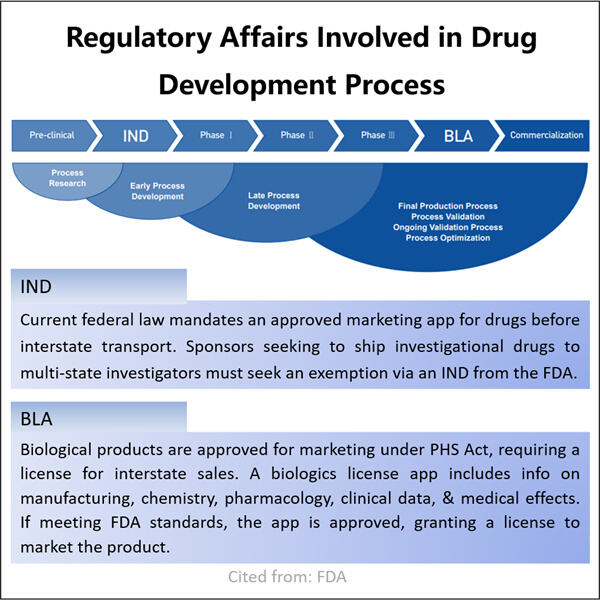
実験室での研究から消費者向け市場への販売に至るまでのバイオ医薬品の道のりは複雑で長期にわたります。このように、科学者たちは新しいバイオ医薬品を見つけるためにはるかに早い段階から研究を始め、疾患を治療する方法を評価します。バイオ医薬品が開発され、成功裏に試験された後でも、消費者への販売前に広範な承認プロセスが行われます。
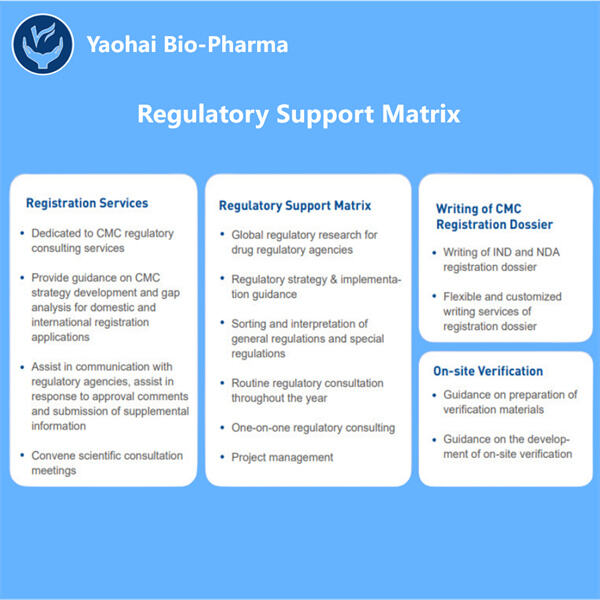
ヤオハイでは、専門家のチームが一流の科学者たちと協力して医薬品の研究開発をサポートしています。彼らは必要な情報を提供するだけでなく、幅広い規制環境についてもガイダンスを提供し、承認のために必要なすべてのデータが提出されるよう確保します。医薬品の承認後も、彼らは規制に基づいて製造および販売するためにさらなる行動を起こします。
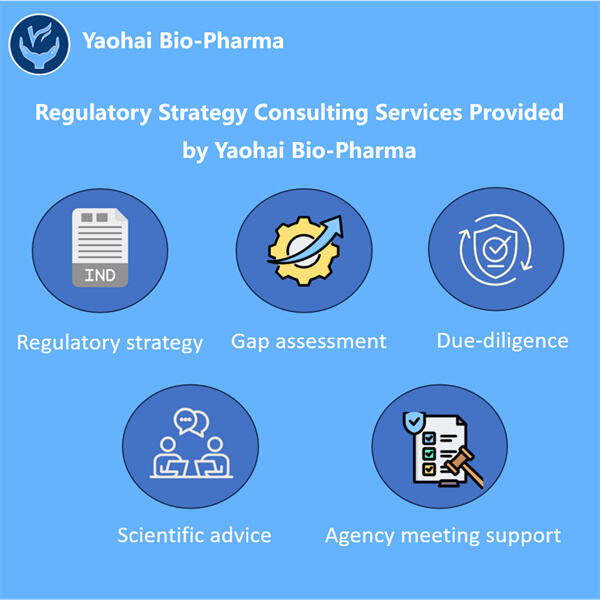
ヤオハイでは、専門家のチームが一流の科学者たちと協力して医薬品の研究開発をサポートしています。彼らは必要な情報を提供するだけでなく、幅広い規制環境についてもガイダンスを提供し、承認のために必要なすべてのデータが提出されるよう確保します。医薬品の承認後も、彼らは規制に基づいて製造および販売するためにさらなる行動を起こします。
Yaohai Bio-Pharmaは、微生物から作られるバイオ医薬品の製造において経験を持っています。私たちはカスタマイズされた研究開発ソリューションと製造サービスを提供し、潜在的なリスクを最小限に抑えます。再構成細胞サブユニット、ワクチン(ペプチドを含む)、成長因子、ホルモン、およびバイオロジクス規制事務など、多様な技術で取り組んできました。私たちは多くの微生物の専門家であり、酵母の細胞外および細胞内分泌(最大15g/Lの収量)や、バクテリアの細胞内可溶性および包含体(最大10g/Lの収量)を取り扱います。また、BSL-2発酵プラットフォームを開発し、細菌ワクチンを作り出しています。私たちは生産プロセスの改善の実績があり、収量を増加させ、コストを削減しています。効率的な技術チームがプロジェクトの迅速かつ高品質な納品を確保します。これにより、独自の製品を市場に早く投入するお手伝いをします。
ヤオハイ バイオファーマは、バイオ医薬品規制事務でトップ10に入る製造業者であり、微生物発酵に特化しています。私たちは、最先端の設備と強力な研究開発製造能力を持つ現代的な施設を設立しました。GMP基準に準拠した微生物の精製および発酵用の5つの生産ライン、ならびにビアルやカートリッジ、プレフィルドシリンジ用の2つの自動充填・仕上げラインが利用可能です。利用可能な発酵規模は100Lから2000Lです。ビアル充填仕様は1ml~25mlをカバーし、プレフィルドカートリッジまたはシリンジの充填仕様は1~3mlです。生産工場はcGMP認証を取得しており、商業用および臨床試験用サンプルの供給が可能です。当工場では大分子を製造し、世界中へ輸出しています。
ヤオハイ・バイオファーマは、品質管理と規制事務を統合したトップ10の微生物CDMOです。当社の品質システムは、現在のGMP基準および国際規制に準拠しています。また、規制の専門家チームは、グローバルな規制フレームワークに精通しており、生物学的製品の上市を加速させます。私たちはトレーサブルな生産プロセス、品質の高い製品、ならびにバイオ医薬品規制事務やEU EMAの要件への適合を確保します。オーストラリアTGAや中国NMPAの要件も満たしています。ヤオハイ・バイオファーマは、ヨーロッパ連合(EU)の認定担当者(QP)による現地審査を無事に通過し、GMPシステムと生産施設が確認されました。さらに、ISO9001品質マネジメントシステムおよびISO14001環境マネジメントシステムの初期認証審査も完了しました。
ヤオハイ バイオファーマは、微生物バイオロジクスの規制事務におけるリーダー企業で、江蘇省に所在しています。当社は、人間、獣医、ペット健康管理に適した微生物由来の治療薬およびワクチンに焦点を当てています。現代的な研究開発および製造技術プラットフォームを保有しており、微生物株のエンジニアリング、細胞バンク、プロセスおよび方法の開発から商業および臨床製造までをカバーし、最先端のソリューションの成功裏の供給を確実にすることができます。私たちは微生物バイオプロセッシング分野で多くの経験を積んでおり、200以上のプロジェクトを成功裡に完了しました。また、US FDAやEU EMAなどの規制対応を顧客に支援するとともに、オーストラリアのTGAや中国のNMPAに対しても支援を行っています。私たちの経験と専門知識により、市場のニーズに迅速に対応し、カスタマイズされたCDMOサービスを提供することができます。