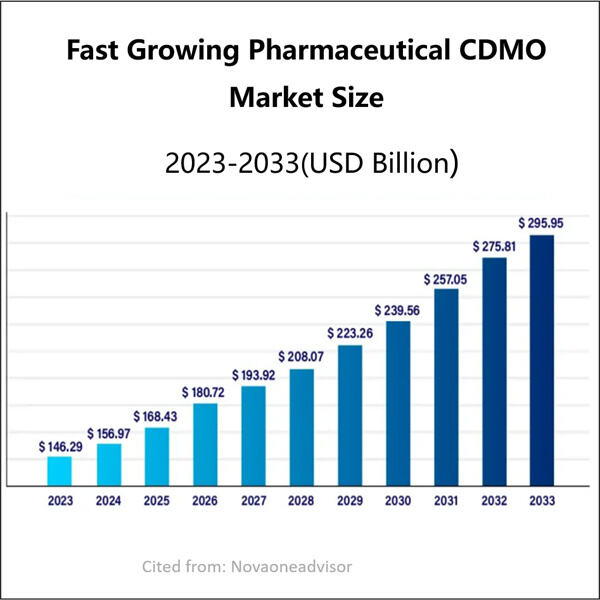
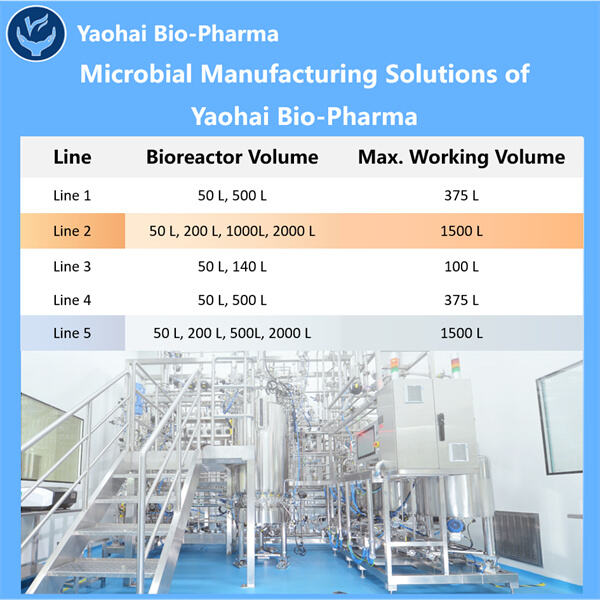
A Yaohai egy fejlődő biogázdasági CDMO vállalat, amely specializálódott gyógyszeres és Biotech alkalmazási megoldások terén. Segítünk nekik abban, hogy kifejlesszék és méretezzék fel (a laborasztaltól a gyárskáláig) két teljesen nem kapcsolatos folyamatot: az egyik hatalmas mennyiségű valódi gyógyszerek előállítására, a másik pedig a formulációk masszteres termelésére, amelyek extrém szükség van rá a további gyógyszereken. Ez a Yaohai Termékek fontos, mivel sokan az emberek függnek ezektől a gyógyszertartalmakaktól a egészségi problémák kezeléséhez. Ez bármennyit tartalmazhat, attól kezdve, hogy találnak-e új gyógyszereket, addig érjenek el, hogy tesztelik, mennyire biztonságosak és hatékonyak az eladás előtt.
A nagy előnnyel a CDMO-val, mint például a Yaohai-val való munkavégzésben annyi, hogy már minden létesítmény, munkaerő és készség rendelkezésre áll a termék fejlesztéséhez alacsonyabb költséggel. Ez alacsonyabb árat eredményez azoknak, akik használják a gyógyszert. Az Mikrobiológiai fermentáció CDMO , amely során hamarabb kerülnek a kezekbe a cégeknek — ahogy sokan mások is gyorsabban használhatják ezeket a fontos gyógyszereket.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN