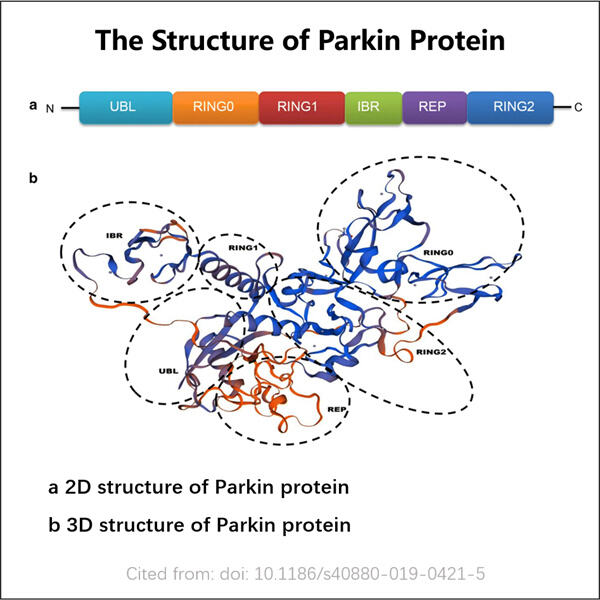
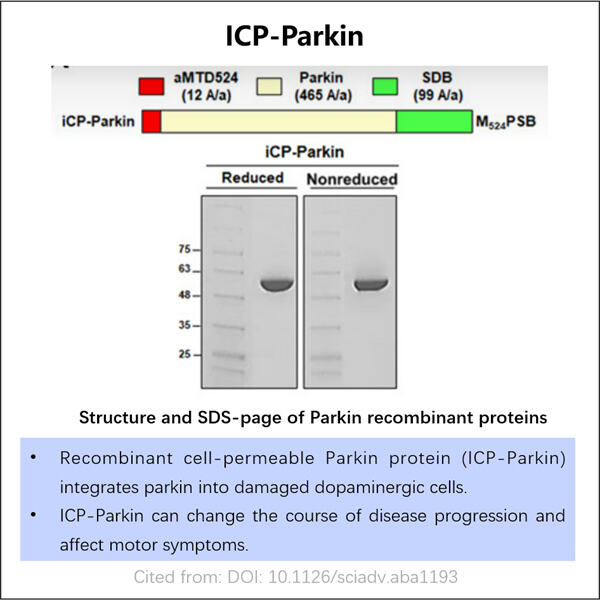
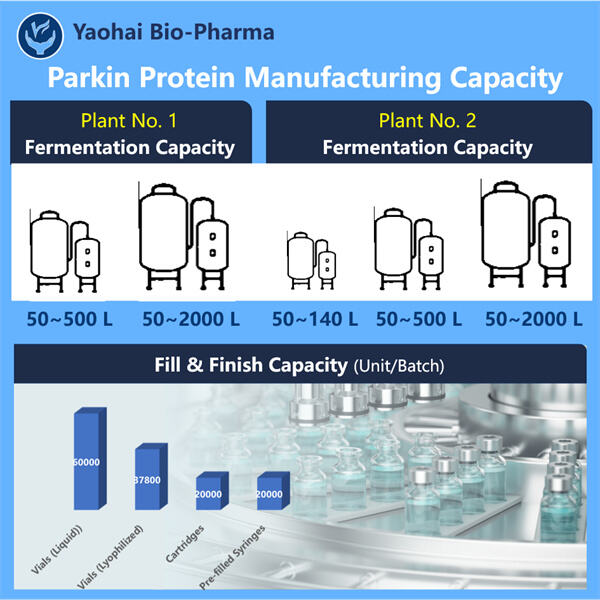
Innovatiewe Produksie Tegnieke vir Rekombinant Parkin-Proteïen
Met hierdie doel in gedagte by Yaohai het ons sommige van die mees uitgewerkte en slimmetjies metodes ontwikkel om herstel parkin-proteïen te produseer. Ontdek waarom ons span wetenskaplikes hierdie ding genetiese ingenieurswese gebruik om 'n spesifieke DNA-reeks te genereer. 'n DNA-reeks wat as riglyne dien om aan selles te sê hoe hulle die parkin-proteïen moet maak. Een ons die DNA voorberei, steek ons dit dalktjie in die selles. Nadat die DNA binne die selle is, begin hulle om daardie instruksies te volg en maak daardie proteïen.
Nadat die rekombinante parkin-selle gegroei het om 'n voldoende kwantiteit rekombinante parkin-proteïen te produseer, sal ons dit uitstrik en purifiseer. Dit is veilig, ons wil seker maak dat die proteïen skoon is en wanneer dit in mediese behandeling gebruik word, is dit veilig. Dit bestaan almal uit verskeie stappe net om seker te maak dat alles reg gedoen is en die proteïen wat geskep word, gereed is vir gebruik deur mense.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN